当前位置:
X-MOL 学术
›
J. Alloys Compd.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A theoretical and experimental study of hexagonal molybdenum trioxide as dual-ion electrode for rechargeable magnesium battery
Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.jallcom.2020.154795 Marta Cabello , Alejandro Medina , Ricardo Alcántara , Francisco Nacimiento , Carlos Pérez-Vicente , José L. Tirado
Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.jallcom.2020.154795 Marta Cabello , Alejandro Medina , Ricardo Alcántara , Francisco Nacimiento , Carlos Pérez-Vicente , José L. Tirado
|
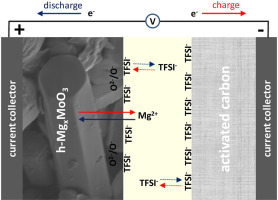
Abstract The hexagonal polytype of molybdenum trioxide (h-MoO3) was prepared through the hydrothermal method. The anisotropic growth of the particles yields to micro rods with prismatic geometry. Theoretical calculations were carried out to simulate the insertion of magnesium in the framework of h-MoO3. The electrochemical behavior of single-phase h-MoO3 in non-aqueous magnesium cells was studied, and for that purpose, Mg metal or activated carbon (A.C.) was used as a counter electrode. This is the first report about the insertion of a divalent cation into h-MoO3. The experimental capacity vs. Mg is only around 20–50 mAh g−1. Nevertheless, whether Mg metal is replaced by A.C. as the counter electrode, the electrochemical behavior of h-MoO3 is improved, and the reversible capacity is about 100 mAh g−1 after 130 cycles. The combination of h-MoO3 and A.C. forms a hybrid or asymmetric electrochemical capacitor. The mechanism of the reaction in the working electrode is more complex than a Mg2+-insertion. Anion (TFSI−) adsorption and redox of oxygen ions in the lattice of h-MoO3 also contribute to the reversible capacity. Consequently, h-MoO3 is a dual-ion electrode material. For higher mass ratio A.C./h-MoO3, the experimental maximum reversible capacity is up to 350 mAh g−1 (equivalent to nominal composition Mg0.94MoO3).
中文翻译:

六方三氧化钼作为可充电镁电池双离子电极的理论与实验研究
摘要 采用水热法制备了六方多晶型三氧化钼(h-MoO3)。颗粒的各向异性生长产生具有棱柱几何形状的微棒。进行理论计算以模拟镁在 h-MoO3 框架中的插入。研究了单相 h-MoO3 在非水镁电池中的电化学行为,为此,使用金属镁或活性炭 (AC) 作为对电极。这是关于将二价阳离子插入 h-MoO3 的第一份报告。与 Mg 相比,实验容量仅为 20-50 mAh g-1 左右。尽管如此,无论用AC代替Mg金属作为对电极,h-MoO3的电化学行为都会得到改善,130次循环后的可逆容量约为100 mAh g-1。h-MoO3 和 AC 的组合形成混合或不对称电化学电容器。工作电极中的反应机制比插入 Mg2+ 更复杂。h-MoO3 晶格中的阴离子 (TFSI-) 吸附和氧离子氧化还原也有助于可逆容量。因此,h-MoO3 是一种双离子电极材料。对于更高质量比的 AC/h-MoO3,实验的最大可逆容量高达 350 mAh g-1(相当于标称成分 Mg0.94MoO3)。
更新日期:2020-08-01
中文翻译:

六方三氧化钼作为可充电镁电池双离子电极的理论与实验研究
摘要 采用水热法制备了六方多晶型三氧化钼(h-MoO3)。颗粒的各向异性生长产生具有棱柱几何形状的微棒。进行理论计算以模拟镁在 h-MoO3 框架中的插入。研究了单相 h-MoO3 在非水镁电池中的电化学行为,为此,使用金属镁或活性炭 (AC) 作为对电极。这是关于将二价阳离子插入 h-MoO3 的第一份报告。与 Mg 相比,实验容量仅为 20-50 mAh g-1 左右。尽管如此,无论用AC代替Mg金属作为对电极,h-MoO3的电化学行为都会得到改善,130次循环后的可逆容量约为100 mAh g-1。h-MoO3 和 AC 的组合形成混合或不对称电化学电容器。工作电极中的反应机制比插入 Mg2+ 更复杂。h-MoO3 晶格中的阴离子 (TFSI-) 吸附和氧离子氧化还原也有助于可逆容量。因此,h-MoO3 是一种双离子电极材料。对于更高质量比的 AC/h-MoO3,实验的最大可逆容量高达 350 mAh g-1(相当于标称成分 Mg0.94MoO3)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号