Chemical Engineering Journal ( IF 15.1 ) Pub Date : 2020-03-24 , DOI: 10.1016/j.cej.2020.124862 James P. Bezzina , Thomas Robshaw , Robert Dawson , Mark D. Ogden
|
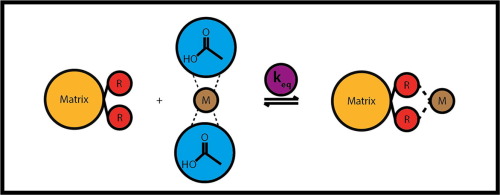
A large predicted increase in population growth and dwindling phosphate resources has led to sewage sludge being an attractive alternative to commercial fertilisers. Among other contaminants, heavy metals are a barrier to unrestricted use of sewage sludge or sewage sludge ash as a phosphate source. This study will focus on the equilibrium isotherm behaviours of Cu2+, Fe2+, Pb2+ and Zn2+ towards C107E, MTS9301 and TP214 resins within acetic acid media, fit to the two parameter Freundlich, Langmuir, Temkin and Dubinin-Radushkevich (D-R) isotherm models. C107E and MTS9301 were both found to have comparable monolayer capacities for Cu2+ and Fe2+ (5±1 vs 4.3±0.7mmol · g−1 and 2.1±0.8 vs 2.3±0.8mmol · g−1 for Cu2+ and Fe2+, respectively). The Freundlich model implied heterogeneous binding for Pb2+ and Zn2+ adsorption to C107E and MTS9301. The monolayer capacities of MTS9301 for lead and zinc were calculated as 2.1±0.2mmol · g−1 and 3±1mmol · g−1, respectively. MTS9301 returned larger D-R free energy values than C107E, with the largest difference being Zn2+, displaying energies of 14.0 and 5.5kJ · mol−1, respectively. TP214 displayed the lowest capacity for metals with Fe2+, Pb2+ and Zn2+ returning D-R energy values closer to physisorption mechanisms (6.0±0.5, 7.1±0.4 and 7.8±0.5kJ · mol−1, respectively), with copper returning a D-R energy relating to chemisorption (17±1kJ · mol−1). Overall, it was observed that the similarity of the C107E functionality to the free acetate anion led to the highest level of hindrance, seconded by the interaction between Fe2+, Pb2+ and Zn2+ to TP214, while copper displayed strong interaction with TP214 and MTS9301 displayed little or no hindrance by the acetate complexes in solution, with solution phase complexes affecting the homogeneity of binding within any ion exchange reaction.
中文翻译:

从合成乙酸浸出液中离子交换去除Cu(II),Fe(II),Pb(II)和Zn(II)的单金属等温线研究
预计人口增长的大量增加和磷酸盐资源的减少已导致污水污泥成为商业肥料的诱人替代品。在其他污染物中,重金属是无限制使用污水污泥或污水污泥灰作为磷酸盐源的障碍。这项研究将着重研究Cu 2 +,Fe 2 +,Pb 2+和Zn 2+在乙酸介质中对C107E,MTS9301和TP214树脂的平衡等温线行为,以适应Freundlich,Langmuir,Temkin和Dubinin-两个参数。 Radushkevich(DR)等温模型。发现C107E和MTS9301都具有相当的Cu 2+和Fe 2+单层容量(5±1对4.3±0.7mmol·g -1Cu 2+和Fe 2+分别为2.1±0.8和2.3±0.8mmol·g -1)。Freundlich模型暗示Pb 2+和Zn 2+吸附到C107E和MTS9301的异质结合。MTS9301的铅和锌的单层容量分别计算为2.1±0.2mmol·g -1和3±1mmol·g -1。MTS9301返回的DR自由能值比C107E大,最大的差为Zn 2+,分别显示14.0和5.5kJ·mol -1的能量。TP214对Fe 2 +,Pb 2+和Zn 2+的金属显示最低的容量返回的DR能量值更接近物理吸附机理(分别为6.0±0.5、7.1±0.4和7.8±0.5kJ·mol -1),而铜返回与化学吸附有关的DR能量(17±1kJ·mol -1)。总体而言,观察到C107E官能团与游离乙酸根阴离子的相似性导致最高水平的阻碍,其次是Fe 2 +,Pb 2+和Zn 2+与TP214的相互作用,而铜表现出强烈的相互作用。 TP214和MTS9301在溶液中的乙酸盐配合物显示很少或没有阻碍,溶液相配合物影响任何离子交换反应中结合的均一性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号