Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-03-23 , DOI: 10.1016/j.cej.2020.124857 Yuan Sun , Wen-Ke Niu , Xiao-Jun Hu , Xiao-Hong Ma , Yu-Jia Sun , Yan Wen
|
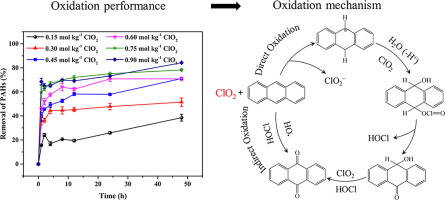
In situ chemical oxidation can be used to remediate soils contaminated with polycyclic aromatic hydrocarbons (PAHs). However, typical oxidation systems are limited by the acidic environment needed for Fenton reactions, cost and soil effects of activated persulfate, and large pores required for ozone or selected oxidation by permanganate. Chlorine dioxide (ClO2) is an environmentally friendly strong oxidant that is highly reactive with PAHs and produces limited halogenated byproducts. In this study, the kinetics, products, and mechanisms of PAH degradation in industrial soil using ClO2 were investigated. The degradation rate was approximately 84.24% for 0.90 mol kg−1 of ClO2; it increased with ClO2 concentration and temperature had little effect. The degradation process was divided into quick (< 1 h) and slow (1–48 h) reaction stages. PAH removal was significantly inhibited at higher pH levels, but > 70% of the PAHs were degraded at a typical soil pH of 5.0–7.0. Chemical pretreatment is favorable for PAH removal during ClO2 oxidation and the degradation rate was passively correlated with the desorbing fraction of the PAHs in the soil. Quenching experiments indicated that HOCl was the most important active species responsible for degradation. Among five representative PAH congeners, the degradation products of anthracene, phenanthrene, and benzo[a]pyrene were all oxygenated products. However, the degradation product of pyrene was the Cl substitution product and fluoranthene oxidation produced ring rupture oxygenated transformation and Cl substitution products. The mechanisms of PAH degradation by ClO2 oxidation include one-electron transfer, HOCl as a second oxidant, and •OH participation.
中文翻译:

用二氧化氯氧化降解工业土壤中的多环芳烃
原位化学氧化可用于修复被多环芳烃(PAH)污染的土壤。但是,典型的氧化系统受到Fenton反应所需的酸性环境,活化过硫酸盐的成本和土壤影响以及臭氧或高锰酸盐选择性氧化所需的大孔的限制。二氧化氯(ClO 2)是一种对环境友好的强氧化剂,与PAHs高度反应并产生有限的卤化副产物。在本研究中,研究了使用ClO 2在工业土壤中PAH降解的动力学,产物和机理。0.90 mol kg -1的ClO 2的降解率约为84.24%。随着ClO 2的增加浓度和温度影响不大。降解过程分为快速(<1小时)和慢速(1-48小时)阶段。在较高的pH值下,PAH的去除受到显着抑制,但是在典型的5.0-7.0的土壤pH下,> 70%的PAHs降解了。化学预处理有利于ClO 2去除PAH氧化和降解速率与土壤中多环芳烃的解吸分数呈被动相关。淬火实验表明,HOCl是引起降解的最重要的活性物质。在五个代表性的PAH同系物中,蒽,菲和苯并[a] re的降解产物均为氧化产物。但是,pyr的降解产物是Cl取代产物,而荧蒽氧化产生的环断裂氧化转变和Cl取代产物。ClO 2氧化降解PAH的机理包括单电子转移,HOCl作为第二种氧化剂和• OH参与。











































 京公网安备 11010802027423号
京公网安备 11010802027423号