Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-03-23 , DOI: 10.1016/j.cej.2020.124864 Weihua Tan , Wei Ren , Chanjuan Wang , Yuanrou Fan , Bin Deng , Heng Lin , Hui Zhang
|
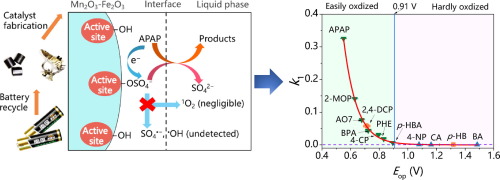
Mn2O3-Fe2O3 catalyst was fabricated from spent alkaline batteries and used for effective elimination of acetaminophen (APAP) by activating peroxymonosulfate (PMS). APAP removal at various initial pH, its degradation pathway and the stability of catalyst were investigated. The ≡Mn(III) was inferred to be the primary active site and ≡Fe(III) could improve the corrosion resistance through intermetallic interactions. Chemical quenching experiments, electron paramagnetic resonance (EPR) spectroscopy, solvent exchange, open circuit potential (OCP) and chronoamperometry tests imply that APAP is oxidized by electron transfer through highly reactive surface-adsorbed PMS. Fourier transform infrared (FTIR), in situ Raman spectroscopy, X-ray photoelectron spectroscopy (XPS) spectra and ionic strength experiments further revealed the interaction between the catalyst and PMS. The Mn2O3-Fe2O3/PMS system exhibits selective oxidation for different contaminants, and linear free energy relationship (LFER) was employed to investigate the effect of structure and properties of pollutant on the reaction kinetics. Pollutant with peak potential (Eop) > 0.91 V, the steady-state OCP in the Mn2O3-Fe2O3/PMS system, is resistant to oxidation; while pollutant with Eop < 0.91 V is prone to be oxidized and there is a good correlation between apparent first-order rate constant (k1) and Eop. The research not only puts in-depth study into PMS activation through environment-friendly Mn-Fe oxides from spent alkaline batteries, but also provides some new insights for nonradical mechanism, selective oxidation and LFER study.
中文翻译:

用废电池基锰铁氧化物活化的过氧单硫酸盐以去除污染物:电子转移机理,选择性氧化和LFER分析
Mn 2 O 3 -Fe 2 O 3催化剂由废碱性电池制成,并用于通过活化过氧单硫酸盐(PMS)有效消除对乙酰氨基酚(APAP)。研究了在各种初始pH条件下APAP的去除,降解途径和催化剂的稳定性。≡Mn(III)被认为是主要的活性部位,而≡Fe(III)可以通过金属间的相互作用提高耐蚀性。化学淬灭实验,电子顺磁共振(EPR)光谱,溶剂交换,开路电势(OCP)和计时电流法测试表明,APAP通过高反应性表面吸附PMS的电子转移而被氧化。原位傅立叶变换红外(FTIR)拉曼光谱,X射线光电子能谱(XPS)光谱和离子强度实验进一步揭示了催化剂与PMS之间的相互作用。Mn 2 O 3 -Fe 2 O 3 / PMS体系对不同的污染物表现出选择性氧化作用,采用线性自由能关系(LFER)研究污染物的结构和性质对反应动力学的影响。Mn 2 O 3 -Fe 2 O 3 / PMS系统中的稳态OCP (峰值电势(E op)> 0.91 V)具有抗氧化性。而污染物与E op<0.91 V容易被氧化,表观的一级速率常数(k 1)与E op之间具有良好的相关性。该研究不仅对废旧碱性电池中环境友好的锰铁氧化物对PMS的活化进行了深入研究,而且为非自由基机理,选择性氧化和LFER研究提供了一些新见识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号