当前位置:
X-MOL 学术
›
Fluid Phase Equilibr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of zwitterionic molecules on ionic association in ethylene oxide-based electrolytes
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.fluid.2020.112572 Manh Tien Nguyen , Qing Shao
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.fluid.2020.112572 Manh Tien Nguyen , Qing Shao
|
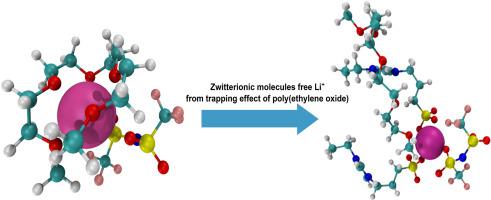
Abstract This work investigates the effect of zwitterionic molecules on ionic association in ethylene oxide (EO)-based electrolytes using molecular dynamics simulations. Zwitterionic molecules can associate with cations and anions because they possess both positively and negatively charged groups. This unique feature can be leveraged to develop electrolytes with high ionic conductivity if we understand how zwitterionic molecules influence ionic associations. We investigate the ionic associations in the electrolytes composed of oligo(ethylene oxide) (EO) (EOx, x = 2, 3, 4, and 5), LiTFSI and zwitterionic molecules containing cationic imidazole group and anionic sulfonate group using molecular dynamics simulations. The analyzed properties include the radial distribution functions between Li+, [TFSI]-, EOx and zwitterionic molecules, the structures and dynamics of Li+-[TFSI]-, Li+- EOx and Li+-zwitterion associations, and the diffusion coefficients of Li+, [TFSI]-, EOx and zwitterionic molecules. The simulation results show two distinct effects of zwitterionic molecules on ionic associations in the electrolytes. First, they could release Li+ from the trapping effect of EOx chains and accelerate Li+ transport. Second, they can associate with Li+ themselves and slow down the Li+ transport. The competition between these two effects relates to the length of the EOx chains. Our simulations suggest that zwitterionic molecules could help manipulate the ionic conductivity of polyethylene oxide electrolytes.
中文翻译:

两性离子分子对环氧乙烷基电解质中离子缔合的影响
摘要 这项工作使用分子动力学模拟研究了两性离子分子对基于环氧乙烷 (EO) 的电解质中离子缔合的影响。两性离子分子可以与阳离子和阴离子缔合,因为它们同时具有带正电荷和带负电荷的基团。如果我们了解两性离子分子如何影响离子结合,就可以利用这种独特的特性来开发具有高离子电导率的电解质。我们使用分子动力学模拟研究了由低聚(环氧乙烷)(EO)(EOx,x = 2、3、4 和 5)、LiTFSI 和含有阳离子咪唑基团和阴离子磺酸盐基团的两性离子分子组成的电解质中的离子缔合。分析的特性包括 Li+、[TFSI]-、EOx 和两性离子分子之间的径向分布函数,Li+-[TFSI]-、Li+- EOx 和 Li+-两性离子缔合的结构和动力学,以及 Li+、[TFSI]-、EOx 和两性离子分子的扩散系数。模拟结果显示两性离子分子对电解质中的离子缔合有两种不同的影响。首先,它们可以从 EOx 链的捕获效应中释放 Li+ 并加速 Li+ 的传输。其次,它们可以与 Li+ 自身结合并减慢 Li+ 的传输。这两种效应之间的竞争与 EOx 链的长度有关。我们的模拟表明,两性离子分子可以帮助控制聚环氧乙烷电解质的离子电导率。模拟结果显示两性离子分子对电解质中的离子缔合有两种不同的影响。首先,它们可以从 EOx 链的捕获效应中释放 Li+ 并加速 Li+ 的传输。其次,它们可以与 Li+ 自身结合并减慢 Li+ 的传输。这两种效应之间的竞争与 EOx 链的长度有关。我们的模拟表明,两性离子分子可以帮助控制聚环氧乙烷电解质的离子电导率。模拟结果显示两性离子分子对电解质中的离子缔合有两种不同的影响。首先,它们可以从 EOx 链的捕获效应中释放 Li+ 并加速 Li+ 的传输。其次,它们可以与 Li+ 自身结合并减慢 Li+ 的传输。这两种效应之间的竞争与 EOx 链的长度有关。我们的模拟表明,两性离子分子可以帮助控制聚环氧乙烷电解质的离子电导率。
更新日期:2020-07-01
中文翻译:

两性离子分子对环氧乙烷基电解质中离子缔合的影响
摘要 这项工作使用分子动力学模拟研究了两性离子分子对基于环氧乙烷 (EO) 的电解质中离子缔合的影响。两性离子分子可以与阳离子和阴离子缔合,因为它们同时具有带正电荷和带负电荷的基团。如果我们了解两性离子分子如何影响离子结合,就可以利用这种独特的特性来开发具有高离子电导率的电解质。我们使用分子动力学模拟研究了由低聚(环氧乙烷)(EO)(EOx,x = 2、3、4 和 5)、LiTFSI 和含有阳离子咪唑基团和阴离子磺酸盐基团的两性离子分子组成的电解质中的离子缔合。分析的特性包括 Li+、[TFSI]-、EOx 和两性离子分子之间的径向分布函数,Li+-[TFSI]-、Li+- EOx 和 Li+-两性离子缔合的结构和动力学,以及 Li+、[TFSI]-、EOx 和两性离子分子的扩散系数。模拟结果显示两性离子分子对电解质中的离子缔合有两种不同的影响。首先,它们可以从 EOx 链的捕获效应中释放 Li+ 并加速 Li+ 的传输。其次,它们可以与 Li+ 自身结合并减慢 Li+ 的传输。这两种效应之间的竞争与 EOx 链的长度有关。我们的模拟表明,两性离子分子可以帮助控制聚环氧乙烷电解质的离子电导率。模拟结果显示两性离子分子对电解质中的离子缔合有两种不同的影响。首先,它们可以从 EOx 链的捕获效应中释放 Li+ 并加速 Li+ 的传输。其次,它们可以与 Li+ 自身结合并减慢 Li+ 的传输。这两种效应之间的竞争与 EOx 链的长度有关。我们的模拟表明,两性离子分子可以帮助控制聚环氧乙烷电解质的离子电导率。模拟结果显示两性离子分子对电解质中的离子缔合有两种不同的影响。首先,它们可以从 EOx 链的捕获效应中释放 Li+ 并加速 Li+ 的传输。其次,它们可以与 Li+ 自身结合并减慢 Li+ 的传输。这两种效应之间的竞争与 EOx 链的长度有关。我们的模拟表明,两性离子分子可以帮助控制聚环氧乙烷电解质的离子电导率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号