当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-Throughput Approach Exploitation: Two-Dimensional Double-Metal Sulfide (M2S2) of Efficient Electrocatalysts for Oxygen Reduction Reaction in Fuel Cells
Energy & Fuels ( IF 5.2 ) Pub Date : 2020-03-22 , DOI: 10.1021/acs.energyfuels.0c00297 Yi Xiao 1 , Li Tang 2
Energy & Fuels ( IF 5.2 ) Pub Date : 2020-03-22 , DOI: 10.1021/acs.energyfuels.0c00297 Yi Xiao 1 , Li Tang 2
Affiliation
|
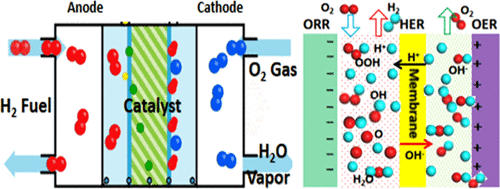
A transition-metal sulfide (M2S2) nanolayer as a catalyst for the oxygen reduction reaction (ORR) has been investigated by the density functional theory (DFT) method to explore the underlying mechanisms of the elementary reaction steps for the ORR process. Both the O2 dissociation and O2 hydrogenation paths are probably possible in the ORR on the M2S2 surface. All of the possible intermediate reaction steps of the ORR are exothermic for O2 hydrogenation. This indicates that the four-electron reaction path (4e– ORR) process is the most favorable path, and it is preferred over the two-electron path (2e– ORR) process. The changes in the reaction free energy diagrams were determined, and these diagrams showed that oxygen hydrogenation (OOH) is the rate-determining step. Meanwhile, different working potentials for our studied catalysts were also considered, and we observed that the double-transition-metal sulfide catalysts are energetically favorable (exothermic) catalysts via a 4e transfer mechanism of the ORR processes. According to the formation energies of the ORR intermediates (*O, *OH, *OOH) and the scaling relations between them on different slabs, the volcano plot for the overpotential of the catalyst is also an important index of the catalytic activities, and we found that a smaller overpotential is appropriate to determine better catalytic activities for the ORR process.
中文翻译:

高通量方法开发:二维双金属硫化物(M 2 S 2),用于燃料电池中的氧还原反应的高效电催化剂
通过密度泛函理论(DFT)方法研究了过渡金属硫化物(M 2 S 2)纳米层作为氧还原反应(ORR)的催化剂,以探索ORR过程基本反应步骤的潜在机理。在M 2 S 2表面上的ORR中,O 2的解离路径和O 2的氢化路径都是可能的。ORR的所有可能的中间反应步骤都是放热的,用于O 2氢化。这表明四电子反应路径(4e – ORR)过程是最有利的路径,它比两电子路径(2e –ORR)过程。确定了反应自由能图中的变化,这些图表明氧氢化(OOH)是决定速率的步骤。同时,还考虑了我们研究的催化剂的不同工作潜力,并且我们观察到,通过ORR工艺的4e转移机理,双过渡金属硫化物催化剂在能量上是有利的(放热)催化剂。根据ORR中间体的形成能(* O,* OH,* OOH)以及它们在不同平板上的比例关系,催化剂超电势的火山图也是催化活性的重要指标,我们发现较小的超电势适合确定ORR过程的更好的催化活性。
更新日期:2020-04-23
中文翻译:

高通量方法开发:二维双金属硫化物(M 2 S 2),用于燃料电池中的氧还原反应的高效电催化剂
通过密度泛函理论(DFT)方法研究了过渡金属硫化物(M 2 S 2)纳米层作为氧还原反应(ORR)的催化剂,以探索ORR过程基本反应步骤的潜在机理。在M 2 S 2表面上的ORR中,O 2的解离路径和O 2的氢化路径都是可能的。ORR的所有可能的中间反应步骤都是放热的,用于O 2氢化。这表明四电子反应路径(4e – ORR)过程是最有利的路径,它比两电子路径(2e –ORR)过程。确定了反应自由能图中的变化,这些图表明氧氢化(OOH)是决定速率的步骤。同时,还考虑了我们研究的催化剂的不同工作潜力,并且我们观察到,通过ORR工艺的4e转移机理,双过渡金属硫化物催化剂在能量上是有利的(放热)催化剂。根据ORR中间体的形成能(* O,* OH,* OOH)以及它们在不同平板上的比例关系,催化剂超电势的火山图也是催化活性的重要指标,我们发现较小的超电势适合确定ORR过程的更好的催化活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号