当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Orientational Switch of the Lipase A Enzyme at the Oil–Water Interface: An Order of Magnitude Increase in Turnover Rate with a Single Surfactant Tag Explained
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2020-03-23 , DOI: 10.1021/acs.jpclett.0c00470 Sudip Das 1 , Sudarshan Behera 1 , Sundaram Balasubramanian 1
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2020-03-23 , DOI: 10.1021/acs.jpclett.0c00470 Sudip Das 1 , Sudarshan Behera 1 , Sundaram Balasubramanian 1
Affiliation
|
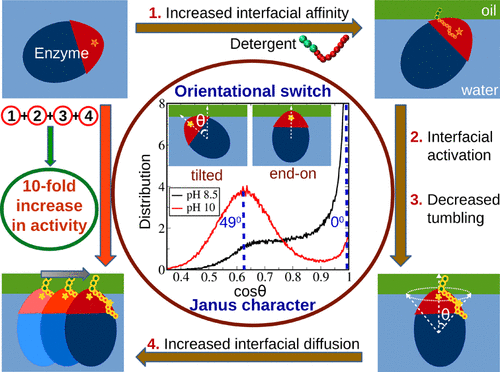
Interfacially active lipases can be immobilized at a biphasic interface to enhance turnover recyclability and to facilitate product separation. Extensive coarse-grained molecular dynamics simulations of lipase A (LipA) from Bacillus subtilis show a bimodal orientational distribution of the enzyme at an oil–water interface, arising from its ellipsoidal Janus particle-like character. The relative orientational preference can be tuned by pH. The simulations rationalize a rare experimental observation of an order of magnitude increase in the turnover rate of this lipase upon its noncovalent tagging by a single surfactant molecule at the interface, compared to its rate in bulk water. The adsorption free energy, the interfacial activation, a decrease in the number of orientational fluctuations, and an increased rate of translational diffusion, to all of which the Janus character of LipA contributes, are the factors responsible for this enhancement. This study can spur further investigations of the Janus behavior of enzymes to enhance their activity as well as to stabilize the biphasic emulsion needed for interfacial catalysis.
中文翻译:

油水界面上脂肪酶A酶的方向转换:解释了单个表面活性剂标记后,周转率增加了一个数量级
界面活性脂肪酶可以固定在两相界面上,以增强周转性和促进产品分离。枯草芽孢杆菌脂肪酶A(LipA)的广泛的粗粒分子动力学模拟显示出酶在油水界面的双峰取向分布,这是由于其椭圆形的Janus颗粒状特征引起的。相对取向偏好可通过pH调节。该模拟合理化了一个罕见的实验观察结果,即该脂肪酶在界面处被单个表面活性剂分子进行非共价标记后,其周转速率与批量水中的速率相比,增加了一个数量级。吸附自由能,界面活化,取向波动数量的减少和平移扩散速率的增加都是LipA的Janus特性做出贡献的原因。
更新日期:2020-04-24
中文翻译:

油水界面上脂肪酶A酶的方向转换:解释了单个表面活性剂标记后,周转率增加了一个数量级
界面活性脂肪酶可以固定在两相界面上,以增强周转性和促进产品分离。枯草芽孢杆菌脂肪酶A(LipA)的广泛的粗粒分子动力学模拟显示出酶在油水界面的双峰取向分布,这是由于其椭圆形的Janus颗粒状特征引起的。相对取向偏好可通过pH调节。该模拟合理化了一个罕见的实验观察结果,即该脂肪酶在界面处被单个表面活性剂分子进行非共价标记后,其周转速率与批量水中的速率相比,增加了一个数量级。吸附自由能,界面活化,取向波动数量的减少和平移扩散速率的增加都是LipA的Janus特性做出贡献的原因。



























 京公网安备 11010802027423号
京公网安备 11010802027423号