当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Free Energy Analysis of a Conformational Change of Heme ABC Transporter BhuUV-T
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2020-03-23 , DOI: 10.1021/acs.jpclett.0c00547 Koichi Tamura 1 , Yuji Sugita 1, 2, 3
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2020-03-23 , DOI: 10.1021/acs.jpclett.0c00547 Koichi Tamura 1 , Yuji Sugita 1, 2, 3
Affiliation
|
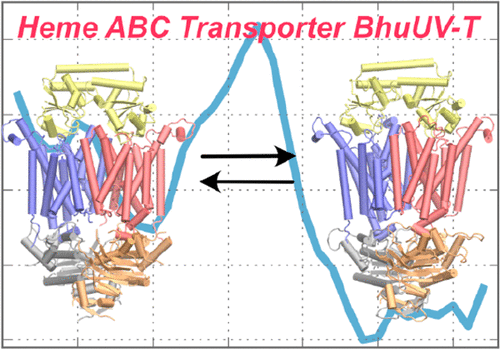
The heme ATP-binding cassette (ABC) transporter BhuUV-T of bacterial pathogen Burkholderia cenocepacia is required to transport heme across the inner cell membrane. The current hypothesis is that the binding of two ATPs to the nucleotide-binding domains of the transporter drives the initial steps of the transport cycle in which the empty transport sites are reoriented from the cytosol to the periplasm. Molecular details are missing because the structure of a key occluded intermediate remains hypothetical. Here we perform molecular simulations to analyze the free energy surface (FES) of the first step of the reorientation, namely the transition from an open inward-facing (IF) transport site to an occluded (Occ) conformation. We have modeled the latter structure in silico in a previous study. A simple annealing procedure removes residual bias originating from non-equilibrium targeted molecular dynamics. The calculated FES reveals the role of the ATPs in inducing the IF → Occ conformational change and validates the modeled Occ conformation.
中文翻译:

血红素 ABC 转运蛋白 BhuUV-T 构象变化的自由能分析
细菌病原体新洋葱伯克霍尔德杆菌的血红素 ATP 结合盒 (ABC) 转运蛋白 BhuUV-T 需要将血红素转运穿过内细胞膜。目前的假设是,两个 ATP 与转运蛋白的核苷酸结合域的结合驱动了转运循环的初始步骤,其中空转运位点从细胞质重新定向到周质。分子细节缺失,因为关键封闭中间体的结构仍然是假设的。在这里,我们进行分子模拟来分析重新定向第一步的自由能表面(FES),即从开放的向内(IF)运输位点到闭塞(Occ)构象的转变。我们在之前的研究中对后一种结构进行了计算机建模。简单的退火程序消除了源自非平衡目标分子动力学的残余偏差。计算的 FES 揭示了 ATP 在诱导 IF → Occ 构象变化中的作用,并验证了建模的 Occ 构象。
更新日期:2020-04-24
中文翻译:

血红素 ABC 转运蛋白 BhuUV-T 构象变化的自由能分析
细菌病原体新洋葱伯克霍尔德杆菌的血红素 ATP 结合盒 (ABC) 转运蛋白 BhuUV-T 需要将血红素转运穿过内细胞膜。目前的假设是,两个 ATP 与转运蛋白的核苷酸结合域的结合驱动了转运循环的初始步骤,其中空转运位点从细胞质重新定向到周质。分子细节缺失,因为关键封闭中间体的结构仍然是假设的。在这里,我们进行分子模拟来分析重新定向第一步的自由能表面(FES),即从开放的向内(IF)运输位点到闭塞(Occ)构象的转变。我们在之前的研究中对后一种结构进行了计算机建模。简单的退火程序消除了源自非平衡目标分子动力学的残余偏差。计算的 FES 揭示了 ATP 在诱导 IF → Occ 构象变化中的作用,并验证了建模的 Occ 构象。











































 京公网安备 11010802027423号
京公网安备 11010802027423号