当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption of Water Molecule on Calcium Fluoride and Magnesium Fluoride Surfaces: A Combined Theoretical and Experimental Study
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.jpcc.0c00713 Jie Cen 1 , Feng Liang 1 , De-Li Chen 1 , Lulu Zhang 1 , Ning Yang 1 , Weidong Zhu 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.jpcc.0c00713 Jie Cen 1 , Feng Liang 1 , De-Li Chen 1 , Lulu Zhang 1 , Ning Yang 1 , Weidong Zhu 1
Affiliation
|
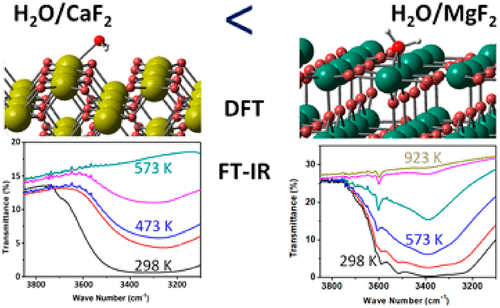
The adsorption and dissociation of H2O molecule on various surfaces of MgF2 and CaF2 have been investigated theoretically by using a dispersion-corrected density functional theory based method. The calculated adsorption energy of H2O molecule on the low index CaF2(110) and CaF2(111) surfaces ranges from −0.20 to −0.50 eV, smaller than those on the low index MgF2(001) and MgF2(110) with values from −0.80 to −1.06 eV. The modeling shows that the dissociation of water molecule is possible on the high index CaF2(210) surface and the low index MgF2(001) and MgF2(110) surfaces as revealed by the calculations that the dissociated states of H2O are thermodynamically more stable. Furthermore, for MgF2(001) and MgF2(110) surfaces, the dissociation process is almost barrierless, suggesting that the OH groups on the surfaces could be formed easily. These theoretical predictions suggest that MgF2 is superior to CaF2 in terms of water adsorption affinity, and high temperatures may be needed to remove the adsorbed water molecules and the OH groups on the surfaces, which were confirmed by the in situ FT-IR spectra of the synthesized MgF2 and CaF2 samples. The broad peak for CaF2 ranging from 3100 to 3600 cm–1 can be completely removed with a temperature of 573 K, while a higher temperature of 923 K is required for MgF2.
中文翻译:

氟化钙和氟化镁表面上水分子的吸附:理论和实验的组合研究
通过使用基于色散校正的密度泛函理论的方法,从理论上研究了H 2 O分子在MgF 2和CaF 2的各种表面上的吸附和解离。H 2 O分子在低折射率CaF 2(110)和CaF 2(111)表面上的吸附能范围为-0.20至-0.50 eV,小于在低折射率MgF 2(001)和MgF 2( 110),其值介于-0.80至-1.06 eV之间。该模型表明水分子可能在高折射率CaF 2(210)表面和低折射率MgF 2(001)和MgF 2表面解离(110)表面通过计算揭示,H 2 O的解离态在热力学上更稳定。此外,对于MgF 2(001)和MgF 2(110)表面,离解过程几乎没有障碍,这表明表面上的OH基很容易形成。这些理论预测表明,MgF 2在吸水亲和力方面优于CaF 2,并且可能需要高温才能去除表面上吸附的水分子和OH基团,这已通过原位FT-IR光谱证实合成的MgF 2和CaF 2样品。CaF 2的宽峰可以在573 K的温度下完全去除3100至3600 cm –1的温度范围,而MgF 2需要更高的923 K温度。
更新日期:2020-03-31
中文翻译:

氟化钙和氟化镁表面上水分子的吸附:理论和实验的组合研究
通过使用基于色散校正的密度泛函理论的方法,从理论上研究了H 2 O分子在MgF 2和CaF 2的各种表面上的吸附和解离。H 2 O分子在低折射率CaF 2(110)和CaF 2(111)表面上的吸附能范围为-0.20至-0.50 eV,小于在低折射率MgF 2(001)和MgF 2( 110),其值介于-0.80至-1.06 eV之间。该模型表明水分子可能在高折射率CaF 2(210)表面和低折射率MgF 2(001)和MgF 2表面解离(110)表面通过计算揭示,H 2 O的解离态在热力学上更稳定。此外,对于MgF 2(001)和MgF 2(110)表面,离解过程几乎没有障碍,这表明表面上的OH基很容易形成。这些理论预测表明,MgF 2在吸水亲和力方面优于CaF 2,并且可能需要高温才能去除表面上吸附的水分子和OH基团,这已通过原位FT-IR光谱证实合成的MgF 2和CaF 2样品。CaF 2的宽峰可以在573 K的温度下完全去除3100至3600 cm –1的温度范围,而MgF 2需要更高的923 K温度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号