Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the Behavior of Perfluorinated Persistent Organic Pollutants (POPs) at Environmentally Relevant Aqueous Interfaces: An Interplay of Hydrophobicity and Hydrogen Bonding
Langmuir ( IF 3.7 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.langmuir.0c00189 Nishith Ghosh , Subhadip Roy , Jahur A. Mondal
Langmuir ( IF 3.7 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.langmuir.0c00189 Nishith Ghosh , Subhadip Roy , Jahur A. Mondal
|
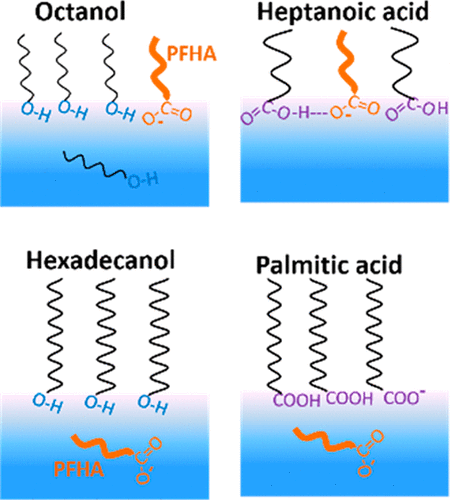
The behavior of perfluorinated persistent organic pollutants (POPs), especially perfluoroalkyl carboxylic and sulfonic acids, at aqueous interfaces is crucial for their transport and speciation in the environment and subsequent immunotoxicity. Here, we investigate the surface prevalence and interfacial interaction of a prototype perfluorinated-POP, perfluoroheptanoic acid (PFHA), with environmentally relevant amphiphiles of varying hydrophobicity and head groups (CnH2n+1–X; n: 8 vs 16; −X: −OH vs −COOH) using interface-selective vibrational sum frequency generation (VSFG) spectroscopy. SFG intensity spectra in the CH- and OH-stretch regions reveal that PFHA prevails at aqueous interfaces that contain amphiphiles of intermediate chain length such as 1-octanol (n = 8) and heptanoic acid (n = 6). PFHA partially expels as well as increases the alkyl chain order of octanol on the water surface. Whereas heptanoic acid, though less hydrophobic than octanol, is retained at the water surface through hydrogen-bonding with the PFHA head group ((PFHA)COO–···HOOC(heptanoic-acid)). Long chain amphiphiles (n = 16) such as hexadecanol and palmitic acid expel PFHA from the water surface regardless of the difference in their head groups. Interestingly, the dangling OH (3710 cm–1) which is diminished at the hydrogenated-amphiphile–water interface is preserved at the perfluorinated-POP–water interface.
中文翻译:

关于全氟持久性有机污染物(POPs)在与环境相关的水界面上的行为:疏水性和氢键的相互作用
全氟持久性有机污染物(POPs),特别是全氟烷基羧酸和磺酸在水界面的行为对于它们在环境中的运输和形成以及随后的免疫毒性至关重要。在这里,我们研究了原型全氟化POP,全氟庚酸(PFHA)与具有不同疏水性和头部基团的环境相关两亲物(C n H 2 n +1 - X ; n:8 vs 16; - X:-OH vs -COOH),使用界面选择性振动和频率生成(VSFG)光谱。在CH和OH拉伸区域的SFG强度光谱显示,PFHA在含有中等链长的两亲物(例如1-辛醇(n = 8)和庚酸(n = 6))的水性界面上占优势。PFHA会部分排出并增加水表面上辛醇的烷基链顺序。庚酸虽然疏水性不如辛醇,但通过与PFHA头基((PFHA) COO – ·· HOOC (庚酸))的氢键结合而保留在水表面。长链两亲分子(ñ= 16),例如十六烷醇和棕榈酸,无论头基团如何不同,都会从水表面排出PFHA。有趣的是,悬空的OH(3710 cm –1)在氢化两亲物-水的界面处减小,而保留在全氟POP-水的界面处。
更新日期:2020-03-31
中文翻译:

关于全氟持久性有机污染物(POPs)在与环境相关的水界面上的行为:疏水性和氢键的相互作用
全氟持久性有机污染物(POPs),特别是全氟烷基羧酸和磺酸在水界面的行为对于它们在环境中的运输和形成以及随后的免疫毒性至关重要。在这里,我们研究了原型全氟化POP,全氟庚酸(PFHA)与具有不同疏水性和头部基团的环境相关两亲物(C n H 2 n +1 - X ; n:8 vs 16; - X:-OH vs -COOH),使用界面选择性振动和频率生成(VSFG)光谱。在CH和OH拉伸区域的SFG强度光谱显示,PFHA在含有中等链长的两亲物(例如1-辛醇(n = 8)和庚酸(n = 6))的水性界面上占优势。PFHA会部分排出并增加水表面上辛醇的烷基链顺序。庚酸虽然疏水性不如辛醇,但通过与PFHA头基((PFHA) COO – ·· HOOC (庚酸))的氢键结合而保留在水表面。长链两亲分子(ñ= 16),例如十六烷醇和棕榈酸,无论头基团如何不同,都会从水表面排出PFHA。有趣的是,悬空的OH(3710 cm –1)在氢化两亲物-水的界面处减小,而保留在全氟POP-水的界面处。











































 京公网安备 11010802027423号
京公网安备 11010802027423号