当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Atomically Dispersed Manganese on a Carbon-Based Material for the Capture of Gaseous Mercury: Mechanisms and Environmental Applications.
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2020-04-08 , DOI: 10.1021/acs.est.9b07524 Jiaxing Li 1 , Haomiao Xu 1 , Yong Liao 1 , Yixiang Qiu 2 , Naiqiang Yan 1 , Zan Qu 1
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2020-04-08 , DOI: 10.1021/acs.est.9b07524 Jiaxing Li 1 , Haomiao Xu 1 , Yong Liao 1 , Yixiang Qiu 2 , Naiqiang Yan 1 , Zan Qu 1
Affiliation
|
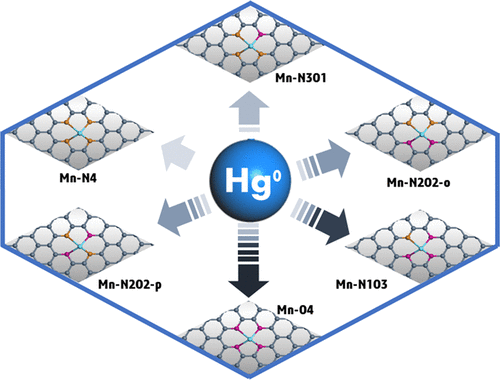
A novel, atomically dispersed carbon-based sorbent was synthesized by anchoring manganese atoms with N atoms for the capture of gaseous elemental Hg (Hg0). Oxygen atoms were also introduced into the synthesis process to adjust the oxidizing ability of the Mn atoms. High-valence Mn (Mn4+) anchored by the O and N atoms (Mn-O/N-C) in the carbon-based materials provided more exposed active sites. The mercury removal efficiency of the composite exceeded 99%. The composite with a Mn loading of 0.9 wt % exhibited high affinity for Hg0, and the capacity for Hg0 adsorption within 275 min at room temperature reached 16.95 mg·g-1. The Mn utilization was ∼56.61%, which is much larger than that of reported Mn-based oxide sorbents. The atomic-level distribution of Mn was well evidenced by aberration-corrected high-angle annular darkfield scanning transmission electron microscopy. Density functional theory calculations were conducted to evaluate the energy for adsorption of Hg0 on Mn-O/N-C. The results indicated that the amount of N and O atoms in the Mn coordination environment determined the Hg0 adsorption energy, and the presence of five optimized Mn adsorption structures in Mn-O/N-C was confirmed by Hg temperature-programmed desorption analysis. These materials may be utilized for mercury removal from disposal sites with high concentrations of mercury, broken mercury-containing lamps, or mercurial thermometers. The strategy of atomic dispersion during synthesis of the materials and adjusting the oxidizing ability in the single-atom strategy may be helpful for the development of environmentally benign functional materials.
中文翻译:

碳基材料上的原子分散锰,用于捕集气态汞:机理和环境应用。
通过将锰原子与N原子锚固来捕获气态元素Hg(Hg0),从而合成了一种新型的原子分散的碳基吸附剂。氧原子也被引入到合成过程中以调节Mn原子的氧化能力。碳基材料中被O和N原子(Mn-O / NC)锚定的高价Mn(Mn4 +)提供了更多的暴露活性位点。复合材料的除汞效率超过99%。Mn负载量为0.9 wt%的复合材料对Hg0具有高亲和力,室温下275 min内对Hg0的吸附能力达到16.95 mg·g-1。锰利用率约为56.61%,远高于已报道的锰基氧化物吸附剂。通过像差校正的高角度环形暗场扫描透射电子显微镜可以很好地证明Mn的原子级分布。进行了密度泛函理论计算,以评估Hg0在Mn-O / NC上的吸附能。结果表明,Mn配位环境中N和O原子的数量决定了Hg0的吸附能,并且通过Hg程序升温脱附分析证实了Mn-O / NC中五个优化的Mn吸附结构的存在。这些材料可用于通过高浓度汞,破碎的含汞灯或水银温度计从处置场所去除汞。
更新日期:2020-04-23
中文翻译:

碳基材料上的原子分散锰,用于捕集气态汞:机理和环境应用。
通过将锰原子与N原子锚固来捕获气态元素Hg(Hg0),从而合成了一种新型的原子分散的碳基吸附剂。氧原子也被引入到合成过程中以调节Mn原子的氧化能力。碳基材料中被O和N原子(Mn-O / NC)锚定的高价Mn(Mn4 +)提供了更多的暴露活性位点。复合材料的除汞效率超过99%。Mn负载量为0.9 wt%的复合材料对Hg0具有高亲和力,室温下275 min内对Hg0的吸附能力达到16.95 mg·g-1。锰利用率约为56.61%,远高于已报道的锰基氧化物吸附剂。通过像差校正的高角度环形暗场扫描透射电子显微镜可以很好地证明Mn的原子级分布。进行了密度泛函理论计算,以评估Hg0在Mn-O / NC上的吸附能。结果表明,Mn配位环境中N和O原子的数量决定了Hg0的吸附能,并且通过Hg程序升温脱附分析证实了Mn-O / NC中五个优化的Mn吸附结构的存在。这些材料可用于通过高浓度汞,破碎的含汞灯或水银温度计从处置场所去除汞。



























 京公网安备 11010802027423号
京公网安备 11010802027423号