当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective α-Allylation of Anilines Enabled by a Combined Palladium and Photoredox Catalytic System
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-24 , DOI: 10.1021/acscatal.0c00871 Hong-Hao Zhang 1 , Jia-Jia Zhao 1 , Shouyun Yu 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-24 , DOI: 10.1021/acscatal.0c00871 Hong-Hao Zhang 1 , Jia-Jia Zhao 1 , Shouyun Yu 1
Affiliation
|
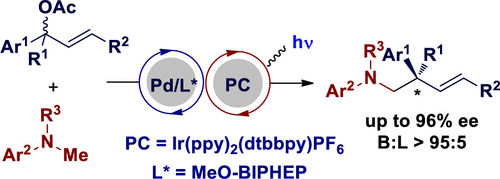
An enantioselective and branch-regioselective α-allylation of N-methyl anilines under dual palladium/photoredox catalysis is described. Readily available N-methyl anilines are used as formal “hard” alkyl nucleophiles without preactivation. Acetic acid is the only side product, which leads to a high atom economy of this reaction. This protocol shows good functional group tolerance and broad scope. A range of chiral homoallylic amines were prepared in moderate to good yields (up to 76%) and excellent regioselectivities (B:L > 95:5 in all cases) and enantioselectivities (up to 96% ee) under mild reaction conditions.
中文翻译:

钯和光氧化还原催化体系共同作用的苯胺对映体选择性α-烯丙基化
描述了在钯/光氧化还原双重催化下N-甲基苯胺的对映选择性和支链区域选择性α-烯丙基化。易于获得的N-甲基苯胺不经预活化即可用作正式的“硬”烷基亲核试剂。乙酸是唯一的副产物,其导致该反应的高原子经济性。该协议显示了良好的功能组耐受性和广泛的范围。在温和的反应条件下,以中等至良好的收率(最高76%),极好的区域选择性(在所有情况下B:L> 95:5)和对映体选择性(最高96%ee)制备了一系列手性均胺。
更新日期:2020-04-23
中文翻译:

钯和光氧化还原催化体系共同作用的苯胺对映体选择性α-烯丙基化
描述了在钯/光氧化还原双重催化下N-甲基苯胺的对映选择性和支链区域选择性α-烯丙基化。易于获得的N-甲基苯胺不经预活化即可用作正式的“硬”烷基亲核试剂。乙酸是唯一的副产物,其导致该反应的高原子经济性。该协议显示了良好的功能组耐受性和广泛的范围。在温和的反应条件下,以中等至良好的收率(最高76%),极好的区域选择性(在所有情况下B:L> 95:5)和对映体选择性(最高96%ee)制备了一系列手性均胺。











































 京公网安备 11010802027423号
京公网安备 11010802027423号