当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhancing Bifunctional Electrocatalytic Activities via Metal d-Band Center Lift Induced by Oxygen Vacancy on the Subsurface of Perovskites
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-24 , DOI: 10.1021/acscatal.0c01104 Hansol Lee 1 , Ohhun Gwon 2 , Keunsu Choi 3 , Linjuan Zhang 4 , Jing Zhou 4 , Jungmin Park 5 , Jung-Woo Yoo 5 , Jian-Qiang Wang 4 , Jun Hee Lee 1 , Guntae Kim 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-24 , DOI: 10.1021/acscatal.0c01104 Hansol Lee 1 , Ohhun Gwon 2 , Keunsu Choi 3 , Linjuan Zhang 4 , Jing Zhou 4 , Jungmin Park 5 , Jung-Woo Yoo 5 , Jian-Qiang Wang 4 , Jun Hee Lee 1 , Guntae Kim 1
Affiliation
|
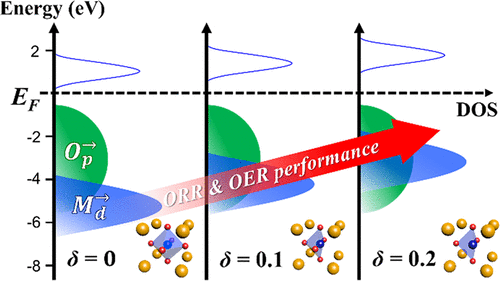
For efficient electrochemical catalysts, several molecular-scale descriptors have been proposed for the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER). Various descriptors of perovskite catalysts have been proposed successfully for understanding either ORR or OER, but previous studies are insufficient to explain and thus boost up both ORR and OER simultaneously due to obstacles such as many different chemical compositions, structures, and metal orbital bands. Therefore, we investigate ORR/OER activities as a function of only oxygen vacancy concentration in perovskite oxides of Sm0.5Sr0.5CoO3−δ (SSC) to check the close relationship between delta (δ) and the electronic structure. Interestingly, the improved performance of both ORR and OER is explained by the change in the oxidation state of the transition metal caused by the increase in oxygen vacancies. Unfortunately, most previous research studies have focused on the effect of only oxygen vacancy (δ) on responsiveness. To confirm this, we performed density functional theory (DFT) analysis to find the more dominant factor on whether the activity descriptor is either δ or oxidation states of transition metals. The DFT analysis reveals that the ORR and OER activities of SSC are simultaneously improved by the reduced gap between d- and p-band centers (ΔEd–p) caused by the raised d-band center (Md). X-ray absorption spectroscopy has provided the exact electronic states of all the transition metals. Here, we report that an important factor of ORR/OER is affected only by the oxidation state of the transition metal in the perovskite oxide, not by the oxygen vacancy concentration.
中文翻译:

通过氧空位引起的钙钛矿表面上的金属d波段中心提升来增强双功能电催化活性
对于有效的电化学催化剂,已经提出了用于氧还原反应(ORR)和氧释放反应(OER)的几种分子尺度描述子。已经成功地提出了钙钛矿催化剂的各种描述来理解ORR或OER,但是由于诸如许多不同的化学组成,结构和金属轨道带的障碍,以前的研究不足以解释并因此同时提高ORR和OER。因此,我们研究了Sr 0.5 Sr 0.5 CoO 3−δ钙钛矿氧化物中ORR / OER活性与仅氧空位浓度的关系(SSC)检查δ(δ)与电子结构之间的紧密关系。有趣的是,ORR和OER的性能提高是由氧空位的增加引起的过渡金属氧化态的变化所解释的。不幸的是,大多数以前的研究集中在仅氧空位(δ)对响应性的影响上。为了证实这一点,我们进行了密度泛函理论(DFT)分析,以发现关于活性描述符是δ还是过渡金属氧化态的更主要因素。DFT分析表明,由于升高的d波段中心(M d)引起的d波段中心和p波段中心(ΔE d–p)之间的间隙减小,SSC的ORR和OER活动同时得到改善)。X射线吸收光谱提供了所有过渡金属的精确电子态。在这里,我们报道ORR / OER的重要因素仅受钙钛矿氧化物中过渡金属的氧化态影响,而不受氧空位浓度的影响。
更新日期:2020-04-23
中文翻译:

通过氧空位引起的钙钛矿表面上的金属d波段中心提升来增强双功能电催化活性
对于有效的电化学催化剂,已经提出了用于氧还原反应(ORR)和氧释放反应(OER)的几种分子尺度描述子。已经成功地提出了钙钛矿催化剂的各种描述来理解ORR或OER,但是由于诸如许多不同的化学组成,结构和金属轨道带的障碍,以前的研究不足以解释并因此同时提高ORR和OER。因此,我们研究了Sr 0.5 Sr 0.5 CoO 3−δ钙钛矿氧化物中ORR / OER活性与仅氧空位浓度的关系(SSC)检查δ(δ)与电子结构之间的紧密关系。有趣的是,ORR和OER的性能提高是由氧空位的增加引起的过渡金属氧化态的变化所解释的。不幸的是,大多数以前的研究集中在仅氧空位(δ)对响应性的影响上。为了证实这一点,我们进行了密度泛函理论(DFT)分析,以发现关于活性描述符是δ还是过渡金属氧化态的更主要因素。DFT分析表明,由于升高的d波段中心(M d)引起的d波段中心和p波段中心(ΔE d–p)之间的间隙减小,SSC的ORR和OER活动同时得到改善)。X射线吸收光谱提供了所有过渡金属的精确电子态。在这里,我们报道ORR / OER的重要因素仅受钙钛矿氧化物中过渡金属的氧化态影响,而不受氧空位浓度的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号