当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rh(I)/Bisoxazolinephosphine-Catalyzed Regio- and Enantioselective Allylic Substitutions
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-23 , DOI: 10.1021/acscatal.0c00712 Wen-Bin Xu 1 , Samir Ghorai 1 , Wenyu Huang 1 , Changkun Li 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-23 , DOI: 10.1021/acscatal.0c00712 Wen-Bin Xu 1 , Samir Ghorai 1 , Wenyu Huang 1 , Changkun Li 1
Affiliation
|
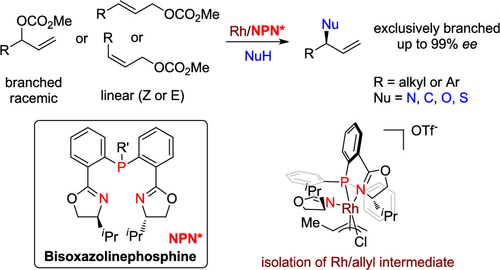
Rhodium(I)/bisoxazolinephosphine combination has been developed as a general catalyst to achieve the dynamic kinetic asymmetric allylation of a variety of nitrogen, carbon, oxygen, and sulfur pronucleophiles from branched racemic allylic carbonates. Exclusive branch-selectivity and up to 99% enantiomeric excess could be obtained under neutral conditions. Linear allylic substrates (both Z and E) could be converted to the same chiral branched products with excellent regio- and enantioselectivities as well. Chiral π-allyl-Rh(III)/NPN intermediate was isolated and characterized to understand the origin of the high selectivities.
中文翻译:

Rh(I)/ Bisoxazolinephosphine催化的区域和对映选择性烯丙基取代
铑(I)/双恶唑啉膦组合已被开发为通用催化剂,可实现支链消旋烯丙基碳酸酯中各种氮,碳,氧和硫亲核试剂的动态动力学不对称烯丙基化。在中性条件下可以获得独家的分支选择性和高达99%的对映体过量。线性烯丙基底物(Z和E)也可以转化为具有出色的区域选择性和对映选择性的相同手性支链产物。分离并表征了手性π-烯丙基-Rh(III)/ NPN中间体,以了解高选择性的起源。
更新日期:2020-04-23
中文翻译:

Rh(I)/ Bisoxazolinephosphine催化的区域和对映选择性烯丙基取代
铑(I)/双恶唑啉膦组合已被开发为通用催化剂,可实现支链消旋烯丙基碳酸酯中各种氮,碳,氧和硫亲核试剂的动态动力学不对称烯丙基化。在中性条件下可以获得独家的分支选择性和高达99%的对映体过量。线性烯丙基底物(Z和E)也可以转化为具有出色的区域选择性和对映选择性的相同手性支链产物。分离并表征了手性π-烯丙基-Rh(III)/ NPN中间体,以了解高选择性的起源。











































 京公网安备 11010802027423号
京公网安备 11010802027423号