当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Circular Permutation of the Native Enzyme-Mediated Cyclization Position in Cyclotides.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-03-30 , DOI: 10.1021/acschembio.9b00996 Bronwyn J Smithies 1 , Yen-Hua Huang 1 , Mark A Jackson 1 , Kuok Yap 1 , Edward K Gilding 1 , Karen S Harris 2 , Marilyn A Anderson 2 , David J Craik 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-03-30 , DOI: 10.1021/acschembio.9b00996 Bronwyn J Smithies 1 , Yen-Hua Huang 1 , Mark A Jackson 1 , Kuok Yap 1 , Edward K Gilding 1 , Karen S Harris 2 , Marilyn A Anderson 2 , David J Craik 1
Affiliation
|
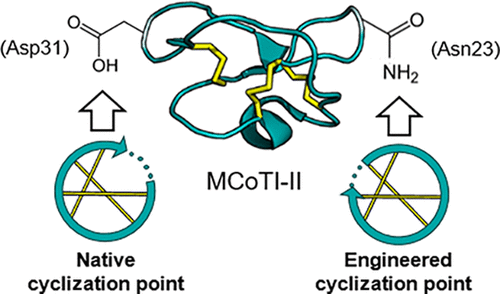
Cyclotides are a class of cyclic disulfide-rich peptides found in plants that have been adopted as a molecular scaffold for pharmaceutical applications due to their inherent stability and ability to penetrate cell membranes. For research purposes, they are usually produced and cyclized synthetically, but there are concerns around the cost and environmental impact of large-scale chemical synthesis. One strategy to improve this is to combine a recombinant production system with native enzyme-mediated cyclization. Asparaginyl endopeptidases (AEPs) are enzymes that can act as peptide ligases in certain plants to facilitate cyclotide maturation. One of these ligases, OaAEP1b, originates from the cyclotide-producing plant, Oldenlandia affinis, and can be produced recombinantly for use in vitro as an alternative to chemical cyclization of recombinant substrates. However, not all engineered cyclotides are compatible with AEP-mediated cyclization because new pharmaceutical epitopes often replace the most flexible region of the peptide, where the native cyclization site is located. Here we redesign a popular cyclotide grafting scaffold, MCoTI-II, to incorporate an AEP cyclization site located away from the usual grafting region. We demonstrate the incorporation of a bioactive peptide sequence in the most flexible region of MCoTI-II while maintaining AEP compatibility, where the two were previously mutually exclusive. We anticipate that our AEP-compatible scaffold, based on the most popular cyclotide for pharmaceutical applications, will be useful in designing bioactive cyclotides that are compatible with AEP-mediated cyclization and will therefore open up the possibility of larger scale enzyme-mediated production of recombinant or synthetic cyclotides alike.
中文翻译:

环氧化物中天然酶介导的环化位置的循环置换。
环核苷酸是植物中发现的一类富含环二硫键的肽,由于其固有的稳定性和穿透细胞膜的能力而被用作药物应用的分子支架。为了研究目的,它们通常是合成生产和环化的,但是人们担心大规模化学合成的成本和环境影响。改善这一状况的一种策略是将重组生产系统与天然酶介导的环化相结合。天冬酰胺基内肽酶(AEP)是可以在某些植物中充当肽连接酶以促进环氧化物成熟的酶。这些连接酶之一OaAEP1b来源于产生环氧化物的植物Oldenlandia affinis,可以重组生产并在体外用作重组底物化学环化的替代方法。但是,并非所有工程化的环肽都与AEP介导的环化反应兼容,因为新的药物表位通常会取代天然环化位点所在的肽段的最柔韧性区域。在这里,我们重新设计了一种流行的环肽接枝支架MCoTI-II,以结合远离常规接枝区域的AEP环化位点。我们证明了在MCoTI-II最灵活的区域中纳入生物活性肽序列,同时保持AEP兼容性,而这两个以前是相互排斥的。我们希望我们的AEP兼容支架基于最流行的用于药物应用的环肽,
更新日期:2020-04-23
中文翻译:

环氧化物中天然酶介导的环化位置的循环置换。
环核苷酸是植物中发现的一类富含环二硫键的肽,由于其固有的稳定性和穿透细胞膜的能力而被用作药物应用的分子支架。为了研究目的,它们通常是合成生产和环化的,但是人们担心大规模化学合成的成本和环境影响。改善这一状况的一种策略是将重组生产系统与天然酶介导的环化相结合。天冬酰胺基内肽酶(AEP)是可以在某些植物中充当肽连接酶以促进环氧化物成熟的酶。这些连接酶之一OaAEP1b来源于产生环氧化物的植物Oldenlandia affinis,可以重组生产并在体外用作重组底物化学环化的替代方法。但是,并非所有工程化的环肽都与AEP介导的环化反应兼容,因为新的药物表位通常会取代天然环化位点所在的肽段的最柔韧性区域。在这里,我们重新设计了一种流行的环肽接枝支架MCoTI-II,以结合远离常规接枝区域的AEP环化位点。我们证明了在MCoTI-II最灵活的区域中纳入生物活性肽序列,同时保持AEP兼容性,而这两个以前是相互排斥的。我们希望我们的AEP兼容支架基于最流行的用于药物应用的环肽,











































 京公网安备 11010802027423号
京公网安备 11010802027423号