当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Glucosylation and O-Acetylation on the Conformation of Shigella flexneri Serogroup 2 O-Antigen Vaccine Targets
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.jpcb.0c01595 Jason Hlozek 1 , Neil Ravenscroft 1 , Michelle M. Kuttel 2
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.jpcb.0c01595 Jason Hlozek 1 , Neil Ravenscroft 1 , Michelle M. Kuttel 2
Affiliation
|
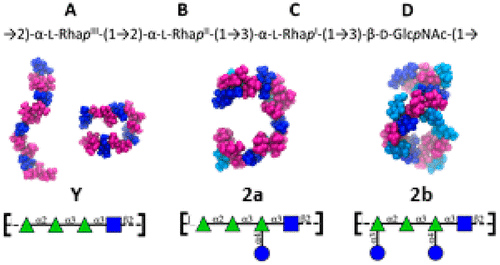
Shigellosis is an enteric disease with high morbidity and mortality, particularly in developing countries. There is currently no licensed vaccine available. Most infection is caused by Shigella flexneri, of which 30 serotypes have been recognized based on O-antigen polysaccharide structure. Almost all S. flexneri serotypes share the same repeating unit backbone (serotype Y), with varying glucosylation, O-acetylation and phosphorylation. The O-antigen is the primary vaccine target; the vaccine valency (and hence cost) can be reduced by cross-protection. Our planned systematic conformational study of S. flexneri starts here with 2a, the dominant cause of infection globally. We employ microsecond molecular dynamics simulations to compare the conformation of the unsubstituted serotype Y backbone with the serogroup 2 O-antigens, to investigate the effect of glucosylation and O-acetylation (O-factor 9) on conformation. We find that serotype Y is highly flexible, whereas glucosylation in 2a restricts flexibility and induces C-curve conformations. Further, the glucose side-chains adopt two distinct conformations, corroborated by the antibody-bound crystal structure data. Additional substitution on O-3 of rhamnose A (whether O-acetylation in 2a or glucosylation in 2b) induces helical conformations. Our results suggest that the O-3-acetylated 2a antigen will elicit cross-protection against 2b, as well as other serotypes containing O-factor 9.
更新日期:2020-03-31











































 京公网安备 11010802027423号
京公网安备 11010802027423号