当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Differential Role of Serines and Threonines in Intracellular Loop 3 and C-Terminal Tail of the Histamine H4 Receptor in β-Arrestin and G Protein-Coupled Receptor Kinase Interaction, Internalization, and Signaling.
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2020-03-16 , DOI: 10.1021/acsptsci.0c00008 Eléonore W E Verweij 1 , Betty Al Araaj 1 , Wimzy R Prabhata 1 , Rudi Prihandoko 2 , Saskia Nijmeijer 1 , Andrew B Tobin 2 , Rob Leurs 1 , Henry F Vischer 1
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2020-03-16 , DOI: 10.1021/acsptsci.0c00008 Eléonore W E Verweij 1 , Betty Al Araaj 1 , Wimzy R Prabhata 1 , Rudi Prihandoko 2 , Saskia Nijmeijer 1 , Andrew B Tobin 2 , Rob Leurs 1 , Henry F Vischer 1
Affiliation
|
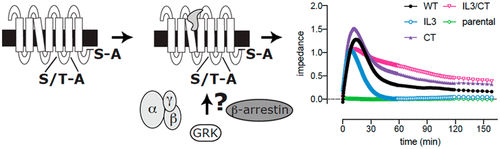
The histamine H4 receptor (H4R) activates Gαi-mediated signaling and recruits β-arrestin2 upon stimulation with histamine. β-Arrestins play a regulatory role in G protein-coupled receptor (GPCR) signaling by interacting with phosphorylated serine and threonine residues in the GPCR C-terminal tail and intracellular loop 3, resulting in receptor desensitization and internalization. Using bioluminescence resonance energy transfer (BRET)-based biosensors, we show that G protein-coupled receptor kinases (GRK) 2 and 3 are more quickly recruited to the H4R than β-arrestin1 and 2 upon agonist stimulation, whereas receptor internalization dynamics toward early endosomes was slower. Alanine-substitution revealed that a serine cluster at the distal end of the H4R C-terminal tail is essential for the recruitment of β-arrestin1/2, and consequently, receptor internalization and desensitization of G protein-driven extracellular-signal-regulated kinase (ERK)1/2 phosphorylation and label-free cellular impedance. In contrast, alanine substitution of serines and threonines in the intracellular loop 3 of the H4R did not affect β-arrestin2 recruitment and receptor desensitization, but reduced β-arrestin1 recruitment and internalization. Hence, β-arrestin recruitment to H4R requires the putative phosphorylated serine cluster in the H4R C-terminal tail, whereas putative phosphosites in the intracellular loop 3 have different effects on β-arrestin1 versus β-arrestin2. Mutation of these putative phosphosites in either intracellular loop 3 or the C-terminal tail did not affect the histamine-induced recruitment of GRK2 and GRK3 but does change the interaction of H4R with GRK5 and GRK6, respectively. Identification of H4R interactions with these proteins is a first step in the understanding how this receptor might be dysregulated in pathophysiological conditions.
中文翻译:

丝氨酸和苏氨酸在β-抑制蛋白和G蛋白偶联的受体激酶相互作用,内在化和信号传导中在组胺H4受体的细胞内环3和C末端尾巴中的不同作用。
组胺H4受体(H4R)在组胺刺激下激活Gαi介导的信号传导并募集β-arrestin2。β-抑制蛋白通过与GPCR C末端尾巴和细胞内环3中的磷酸化丝氨酸和苏氨酸残基相互作用,在G蛋白偶联受体(GPCR)信号传导中发挥调节作用,导致受体脱敏和内在化。使用基于生物发光共振能量转移(BRET)的生物传感器,我们显示在激动剂刺激下,G蛋白偶联受体激酶(GRK)2和3比β-arrestin1和2更快速地被募集到H4R,而受体内化动力学朝着早期方向发展。内体慢。丙氨酸替代显示H4R C末端尾部远端的丝氨酸簇对于募集β-arrestin1/ 2至关重要,因此,G蛋白驱动的细胞外信号调节激酶(ERK)1/2磷酸化和无标记细胞阻抗的受体内化和脱敏。相反,H4R的细胞内环3中丝氨酸和苏氨酸的丙氨酸取代不影响β-arrestin2募集和受体脱敏,但减少了β-arrestin1募集和内在化。因此,β-arrestin募集到H4R需要在H4R C末端尾部假定磷酸化的丝氨酸簇,而细胞内环3中的假定磷酸位点对β-arrestin1和β-arrestin2的作用不同。这些推定的磷酸位点在细胞内环3或C末端尾部的突变不会影响组胺诱导的GRK2和GRK3募集,但确实会改变H4R与GRK5和GRK6的相互作用。
更新日期:2020-04-23
中文翻译:

丝氨酸和苏氨酸在β-抑制蛋白和G蛋白偶联的受体激酶相互作用,内在化和信号传导中在组胺H4受体的细胞内环3和C末端尾巴中的不同作用。
组胺H4受体(H4R)在组胺刺激下激活Gαi介导的信号传导并募集β-arrestin2。β-抑制蛋白通过与GPCR C末端尾巴和细胞内环3中的磷酸化丝氨酸和苏氨酸残基相互作用,在G蛋白偶联受体(GPCR)信号传导中发挥调节作用,导致受体脱敏和内在化。使用基于生物发光共振能量转移(BRET)的生物传感器,我们显示在激动剂刺激下,G蛋白偶联受体激酶(GRK)2和3比β-arrestin1和2更快速地被募集到H4R,而受体内化动力学朝着早期方向发展。内体慢。丙氨酸替代显示H4R C末端尾部远端的丝氨酸簇对于募集β-arrestin1/ 2至关重要,因此,G蛋白驱动的细胞外信号调节激酶(ERK)1/2磷酸化和无标记细胞阻抗的受体内化和脱敏。相反,H4R的细胞内环3中丝氨酸和苏氨酸的丙氨酸取代不影响β-arrestin2募集和受体脱敏,但减少了β-arrestin1募集和内在化。因此,β-arrestin募集到H4R需要在H4R C末端尾部假定磷酸化的丝氨酸簇,而细胞内环3中的假定磷酸位点对β-arrestin1和β-arrestin2的作用不同。这些推定的磷酸位点在细胞内环3或C末端尾部的突变不会影响组胺诱导的GRK2和GRK3募集,但确实会改变H4R与GRK5和GRK6的相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号