当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantum Chemistry Study on Gas Reaction Mechanism in AlN MOVPE Growth
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-04-02 , DOI: 10.1021/acs.jpca.9b11817 Hong Zhang 1 , Ran Zuo 1 , Tingting Zhong 1 , Lian Zhang 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-04-02 , DOI: 10.1021/acs.jpca.9b11817 Hong Zhang 1 , Ran Zuo 1 , Tingting Zhong 1 , Lian Zhang 1
Affiliation
|
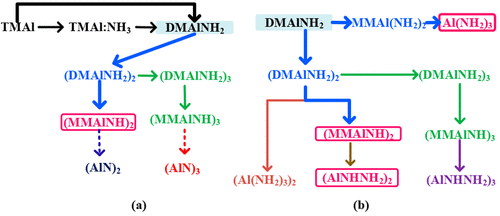
By using the density functional theory of quantum chemistry, the gas reaction mechanism in the AlN MOVPE process has been investigated, especially after the amide DMAlNH2 formation. Two reaction paths are distinguished after the amide DMAlNH2 formation and oligomerization: the intramolecular path and the intermolecular path, both involved with methane elimination. By inspections of the changes of the Gibbs energy ΔG between products and reactants, as well as the Gibbs energy of activation divided by RT, ΔG*/RT, to account for thermal activation at different temperatures, the most probable gas reaction paths, and gas products for AlN thin film growth are determined both thermodynamically and kinetically. Our results indicate that under metal organic vapor phase epitaxy condition, for the intramolecular path, (MMAlNH)2 is the most probable gas reaction products; for the intermolecular path, both Al(NH2)3 and (AlNHNH2)2 are the most probable gas reaction products. We also prove that (AlN)2 and (AlN)3 clusters are thermodynamically unfavored in the gas phase.
中文翻译:

AlN MOVPE生长中气体反应机理的量子化学研究
通过使用量子化学的密度泛函理论,研究了AlN MOVPE过程中的气体反应机理,特别是在酰胺DMAlNH 2形成之后。在酰胺DMA1NH 2的形成和低聚之后,区分了两个反应路径:分子内路径和分子间路径,两者均与甲烷消除有关。通过检查产物和反应物之间的吉布斯能量ΔG的变化,以及活化的吉布斯能量除以RT,ΔG * / RT为了说明在不同温度下的热活化,最可能的气体反应路径和用于AlN薄膜生长的气体产物是通过热力学和动力学方法确定的。我们的结果表明,在金属有机气相外延条件下,对于分子内路径而言,(MMAlNH)2是最可能的气体反应产物。对于分子间路径,Al(NH 2)3和(AlNHNH 2)2都是最可能的气体反应产物。我们还证明了(AlN)2和(AlN)3团簇在气相中在热力学上是不利的。
更新日期:2020-04-03
中文翻译:

AlN MOVPE生长中气体反应机理的量子化学研究
通过使用量子化学的密度泛函理论,研究了AlN MOVPE过程中的气体反应机理,特别是在酰胺DMAlNH 2形成之后。在酰胺DMA1NH 2的形成和低聚之后,区分了两个反应路径:分子内路径和分子间路径,两者均与甲烷消除有关。通过检查产物和反应物之间的吉布斯能量ΔG的变化,以及活化的吉布斯能量除以RT,ΔG * / RT为了说明在不同温度下的热活化,最可能的气体反应路径和用于AlN薄膜生长的气体产物是通过热力学和动力学方法确定的。我们的结果表明,在金属有机气相外延条件下,对于分子内路径而言,(MMAlNH)2是最可能的气体反应产物。对于分子间路径,Al(NH 2)3和(AlNHNH 2)2都是最可能的气体反应产物。我们还证明了(AlN)2和(AlN)3团簇在气相中在热力学上是不利的。









































 京公网安备 11010802027423号
京公网安备 11010802027423号