当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fluoride Migration Catalysis Enables Simple, Stereoselective, and Iterative Glycosylation
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-03-24 , DOI: 10.1021/jacs.0c03165 Girish C Sati 1 , Joshua L Martin 1 , Yishu Xu 1 , Tanmay Malakar 1 , Paul M Zimmerman 1 , John Montgomery 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-03-24 , DOI: 10.1021/jacs.0c03165 Girish C Sati 1 , Joshua L Martin 1 , Yishu Xu 1 , Tanmay Malakar 1 , Paul M Zimmerman 1 , John Montgomery 1
Affiliation
|
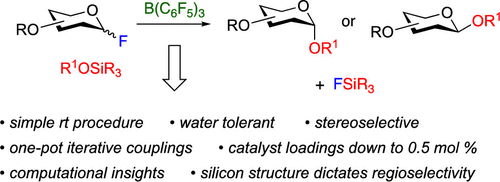
Challenges in the assembly of glycosidic bonds in oligosaccharides and glycoconjugates pose a bottleneck in enabling the remarkable promise of advances in the glycosciences. Here, we report a strategy that applies unique features of highly electrophilic boron catalysts, such as tris(pentafluorophenyl)borane, in addressing a number of the current limitations of methods in glycoside synthesis. This approach utilizes glycosyl fluoride donors and silyl ether acceptors while tolerating the Lewis basic environment found in carbohydrates. The method can be carried out at room temperature using air- and moisture stable forms of the catalyst, with loadings as low as 0.5 mol %. These characteristics enable a wide array of glycosylation patterns to be accessed, including all C1-C2 stereochemical relationships in the glucose, mannose and rhamnose series. This method allows one-pot, iterative glycosylations to generate oligosaccharides directly from monosaccharide building blocks. These advances enable the rapid and experimentally straightforward preparation of complex oligosaccharide units from simple building blocks.
中文翻译:

氟化物迁移催化实现简单、立体选择性和迭代糖基化
寡糖和糖缀合物中糖苷键组装方面的挑战是实现糖科学进步的显着前景的瓶颈。在这里,我们报告了一种策略,该策略应用了高亲电性硼催化剂(如三(五氟苯基)硼烷)的独特特性,以解决目前糖苷合成方法的一些局限性。这种方法利用糖基氟供体和甲硅烷基醚受体,同时耐受碳水化合物中发现的路易斯碱性环境。该方法可以在室温下使用空气和水分稳定形式的催化剂进行,负载量低至 0.5 mol%。这些特征使得可以访问广泛的糖基化模式,包括葡萄糖、甘露糖和鼠李糖系列中的所有 C1-C2 立体化学关系。这种方法允许一锅、迭代糖基化直接从单糖构建块生成寡糖。这些进步使得能够从简单的构建单元中快速且通过实验直接制备复杂的寡糖单元。
更新日期:2020-03-24
中文翻译:

氟化物迁移催化实现简单、立体选择性和迭代糖基化
寡糖和糖缀合物中糖苷键组装方面的挑战是实现糖科学进步的显着前景的瓶颈。在这里,我们报告了一种策略,该策略应用了高亲电性硼催化剂(如三(五氟苯基)硼烷)的独特特性,以解决目前糖苷合成方法的一些局限性。这种方法利用糖基氟供体和甲硅烷基醚受体,同时耐受碳水化合物中发现的路易斯碱性环境。该方法可以在室温下使用空气和水分稳定形式的催化剂进行,负载量低至 0.5 mol%。这些特征使得可以访问广泛的糖基化模式,包括葡萄糖、甘露糖和鼠李糖系列中的所有 C1-C2 立体化学关系。这种方法允许一锅、迭代糖基化直接从单糖构建块生成寡糖。这些进步使得能够从简单的构建单元中快速且通过实验直接制备复杂的寡糖单元。



























 京公网安备 11010802027423号
京公网安备 11010802027423号