当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ligand-Enabled mono-Selective β-C(sp3)–H Acyloxylation of Free Carboxylic Acids Using a Practical Oxidant
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-23 , DOI: 10.1021/jacs.0c01214 Zhe Zhuang 1 , Alastair N Herron 1 , Zhoulong Fan 1 , Jin-Quan Yu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-23 , DOI: 10.1021/jacs.0c01214 Zhe Zhuang 1 , Alastair N Herron 1 , Zhoulong Fan 1 , Jin-Quan Yu 1
Affiliation
|
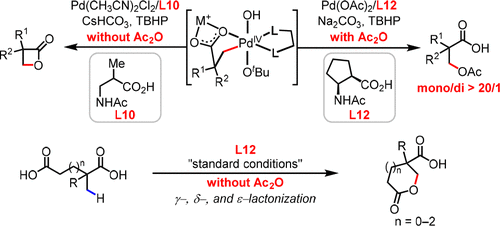
The development of C-H activation reactions that use inexpensive and practical oxidants remains a significant challenge. Until our recent disclosure of the β-lactonization of free aliphatic acids, the use of peroxides in C-H activation reactions directed by weakly coordinating native functional groups was unreported. Herein we report C(sp3)-H β-acetoxylation and γ-, δ-, and ε-lactonization reactions of free carboxylic acids enabled by a novel cyclopentane-based mono-N-protected β-amino acid (MPAA) ligand. Notably, tert-butyl hydrogen peroxide (TBHP) is used as the sole oxidant for these reactions. This reaction has several key advantages over other C-H activation protocols: (1) exclusive mono-selectivity was observed in the presence of two α-methyl groups; (2) aliphatic carboxylic acids containing α-hydrogens are compatible with this protocol; (3) lactonization of free acids, affording γ-, δ-, or ε-lactones, has been achieved for the first time.
中文翻译:

使用实用氧化剂对游离羧酸进行配体激活的单选择性 β-C(sp3)-H 酰氧基化
开发使用廉价且实用的氧化剂的 CH 活化反应仍然是一个重大挑战。在我们最近公开游离脂肪酸的 β-内酯化之前,过氧化物在由弱配位天然官能团指导的 CH 活化反应中的使用还没有报道。在此,我们报告了由新型环戊烷基单-N-保护的 β-氨基酸 (MPAA) 配体实现的游离羧酸的 C(sp3)-H β-乙酰氧基化和 γ-、δ- 和 ε-内酯化反应。值得注意的是,叔丁基过氧化氢 (TBHP) 被用作这些反应的唯一氧化剂。与其他 CH 活化方案相比,该反应具有几个关键优势:(1) 在存在两个 α-甲基基团的情况下观察到唯一的单选择性;(2) 含有α-氢的脂肪族羧酸与本协议兼容;
更新日期:2020-03-23
中文翻译:

使用实用氧化剂对游离羧酸进行配体激活的单选择性 β-C(sp3)-H 酰氧基化
开发使用廉价且实用的氧化剂的 CH 活化反应仍然是一个重大挑战。在我们最近公开游离脂肪酸的 β-内酯化之前,过氧化物在由弱配位天然官能团指导的 CH 活化反应中的使用还没有报道。在此,我们报告了由新型环戊烷基单-N-保护的 β-氨基酸 (MPAA) 配体实现的游离羧酸的 C(sp3)-H β-乙酰氧基化和 γ-、δ- 和 ε-内酯化反应。值得注意的是,叔丁基过氧化氢 (TBHP) 被用作这些反应的唯一氧化剂。与其他 CH 活化方案相比,该反应具有几个关键优势:(1) 在存在两个 α-甲基基团的情况下观察到唯一的单选择性;(2) 含有α-氢的脂肪族羧酸与本协议兼容;











































 京公网安备 11010802027423号
京公网安备 11010802027423号