当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dehydrogenation of Primary Alkyl Azides to Nitriles Catalyzed by Pincer Iridium/Ruthenium Complexes
ChemCatChem ( IF 3.8 ) Pub Date : 2020-03-21 , DOI: 10.1002/cctc.202000260 Lan Gan 1 , Xiangqing Jia 1 , Huaquan Fang 1 , Guixia Liu 1 , Zheng Huang 1, 2, 3
ChemCatChem ( IF 3.8 ) Pub Date : 2020-03-21 , DOI: 10.1002/cctc.202000260 Lan Gan 1 , Xiangqing Jia 1 , Huaquan Fang 1 , Guixia Liu 1 , Zheng Huang 1, 2, 3
Affiliation
|
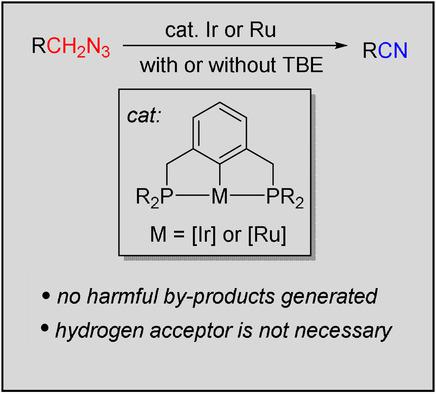
Pincer metal complexes exhibit superior catalytic activity in the dehydrogenation of plain alkanes, but find limited application in the dehydrogenation of functionalized organic molecules. Starting from easily accessible primary alkyl azides, here we report an efficient dehydrogenation of azides to nitriles using pincer iridium or ruthenium complexes as the catalysts. This method offers a route to cyanide‐free preparation of nitriles without carbon chain elongation and without the use of strong oxidants. Both benzyl and linear aliphatic azides can be dehydrogenated with tert‐butylethylene as the hydrogen acceptor to afford nitriles in moderate to high yields. Various functional groups can be tolerated, and the H−C−C−H bond dehydrogenation does not occur for linear alkyl azide substrates. Furthermore, the pincer Ir catalytic system was found to catalyze the direct azide dehydrogenation without the use of a sacrificial hydrogen acceptor.
中文翻译:

钳式铱/钌配合物催化将烷基叠氮化物脱氢成腈
夹钳金属络合物在纯链烷烃的脱氢中显示出优异的催化活性,但在功能化有机分子的脱氢中发现有限的应用。从容易获得的伯烷基叠氮化物开始,在这里我们报告了使用夹钳铱或钌络合物作为催化剂将叠氮化物有效脱氢为腈。该方法提供了一种无需氰化物即可制备腈的方法,该方法无需碳链延长,也无需使用强氧化剂。两个苄基和直链脂肪族叠氮化物可以与脱氢叔-丁基乙烯作为氢受体以中等至高产率提供腈。可以容忍各种官能团,并且对于线性烷基叠氮化物底物不会发生HC-CH键脱氢。此外,发现在不使用牺牲氢受体的情况下,夹钳式Ir催化体系可催化直接叠氮化物脱氢。
更新日期:2020-03-21
中文翻译:

钳式铱/钌配合物催化将烷基叠氮化物脱氢成腈
夹钳金属络合物在纯链烷烃的脱氢中显示出优异的催化活性,但在功能化有机分子的脱氢中发现有限的应用。从容易获得的伯烷基叠氮化物开始,在这里我们报告了使用夹钳铱或钌络合物作为催化剂将叠氮化物有效脱氢为腈。该方法提供了一种无需氰化物即可制备腈的方法,该方法无需碳链延长,也无需使用强氧化剂。两个苄基和直链脂肪族叠氮化物可以与脱氢叔-丁基乙烯作为氢受体以中等至高产率提供腈。可以容忍各种官能团,并且对于线性烷基叠氮化物底物不会发生HC-CH键脱氢。此外,发现在不使用牺牲氢受体的情况下,夹钳式Ir催化体系可催化直接叠氮化物脱氢。










































 京公网安备 11010802027423号
京公网安备 11010802027423号