当前位置:
X-MOL 学术
›
Int. J. Energy Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigation of the iodine species on platinum catalyst used as hydrogen oxidation
International Journal of Energy Research ( IF 4.3 ) Pub Date : 2020-03-21 , DOI: 10.1002/er.5345 Hee‐Jung Im 1 , Keun Man Park 2 , Jei‐Won Yeon 3
International Journal of Energy Research ( IF 4.3 ) Pub Date : 2020-03-21 , DOI: 10.1002/er.5345 Hee‐Jung Im 1 , Keun Man Park 2 , Jei‐Won Yeon 3
Affiliation
|
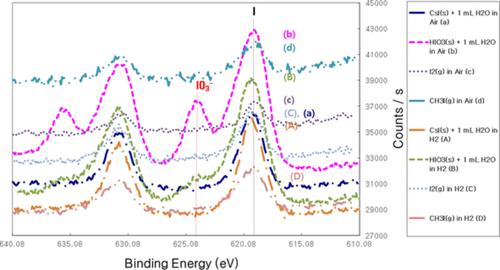
Hydrogen can be oxidized by recombination with atmospheric oxygen over a catalyst, such as Pt/Al2O3, at a temperature much lower than that required for thermal oxidation generating heat and water vapor. However, there is potential for the passive autocatalytic recombiner to generate volatile forms of iodine, namely molecular iodine, by catalytic dissociation of various iodine compounds too. The dissociated atomic iodine on the surfaces of the Pt/Al2O3 catalyst is mainly measured at ambient temperature as I 3d peaks in X‐ray photoelectron spectroscopy. It seems that dissociation of iodine compounds on the catalyst surfaces and then production of gaseous I2 by recombination of the dissociated iodine occur prior to the oxidation or reduction of iodine compounds under air or H2. Atomic iodine is predominant on the surfaces of the catalyst in H2 and air conditions, and it is stable even with exposure to H2O. Adsorbed iodine prevents the adsorption of H2 or O2 for recombination to H2O, but it can be desorbed completely at a high temperature.
中文翻译:

铂催化氢氧化反应中碘种类的研究
氢气可以通过在催化剂(例如Pt / Al 2 O 3)上与大气中的氧重新混合,而氧化的温度要比热氧化产生热量和水蒸气所需的温度低得多。但是,被动自催化重组器也可能通过各种碘化合物的催化离解而生成挥发性形式的碘,即分子碘。Pt / Al 2 O 3催化剂表面的离解原子碘主要在环境温度下测量,这是X射线光电子能谱中的I 3d峰。看来碘化合物在催化剂表面解离,然后生成气态I 2在空气或H 2下,碘化物的氧化或还原之前,发生离解的碘的重组。在H 2和空气条件下,原子碘主要存在于催化剂的表面,即使暴露于H 2 O也能保持稳定。吸附的碘会阻止H 2或O 2吸附而重组为H 2 O,但它可以在高温下完全解吸。
更新日期:2020-03-21
中文翻译:

铂催化氢氧化反应中碘种类的研究
氢气可以通过在催化剂(例如Pt / Al 2 O 3)上与大气中的氧重新混合,而氧化的温度要比热氧化产生热量和水蒸气所需的温度低得多。但是,被动自催化重组器也可能通过各种碘化合物的催化离解而生成挥发性形式的碘,即分子碘。Pt / Al 2 O 3催化剂表面的离解原子碘主要在环境温度下测量,这是X射线光电子能谱中的I 3d峰。看来碘化合物在催化剂表面解离,然后生成气态I 2在空气或H 2下,碘化物的氧化或还原之前,发生离解的碘的重组。在H 2和空气条件下,原子碘主要存在于催化剂的表面,即使暴露于H 2 O也能保持稳定。吸附的碘会阻止H 2或O 2吸附而重组为H 2 O,但它可以在高温下完全解吸。











































 京公网安备 11010802027423号
京公网安备 11010802027423号