Chemical Physics ( IF 2.0 ) Pub Date : 2020-03-21 , DOI: 10.1016/j.chemphys.2020.110763 D. Yancheva , S. Stoyanov , K. Anichina , S. Nikolova , E. Velcheva , B. Stamboliyska
|
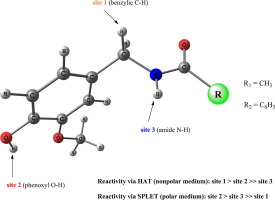
Two capsaicin analogues – N-(4-hydroxy-3-methoxybenzyl)acetamide and N-(4-hydroxy-3-methoxybenzyl)benzamide, were studied by DFT methods in order to estimate their ability to act as antioxidants. A comparative study on the stability of benzylic, phenoxyl and amide radicals has outlined the most reactive site for hydrogen atom abstraction and proton transfer. The enthalpies related to the formation of those species were modeled in gas phase, benzene, DMSO and water in order to determine the most probable mechanism of antioxidant action in polar and nonpolar medium. The formation of phenoxyl anion, energetically favoured in polar medium, was investigated by infrared spectroscopy methods based on the conversion of the benzamide derivative.
中文翻译:

辣椒素衍生物及其阴离子中间体在清除自由基中的红外光谱和计算研究相结合
通过DFT方法研究了两种辣椒素类似物– N-(4-羟基-3-甲氧基苄基)乙酰胺和N-(4-羟基-3-甲氧基苄基)苯甲酰胺,以评估其作为抗氧化剂的能力。对苄基,苯氧基和酰胺基的稳定性进行的比较研究概述了氢原子提取和质子转移的最活泼的部位。在气相,苯,DMSO和水中模拟与这些物质形成相关的焓,以确定在极性和非极性介质中最可能的抗氧化剂作用机理。基于苯甲酰胺衍生物的转化率,通过红外光谱法研究了在极性介质中大力支持的苯氧基阴离子的形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号