Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-03-22 , DOI: 10.1016/j.cej.2020.124839 Lei Zhou , Qing Zhao , Xuerui Yang , Corinne Ferronato , Jean-Marc Chovelon , Mohamad Sleiman , Claire Richard
|
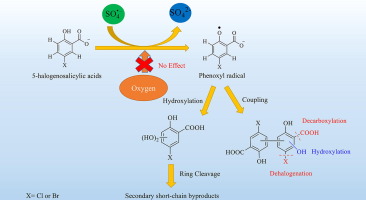
In the present study, we investigated the degradation kinetics and transformation pathways of two 5-halogenosalicylic acids (5XSAs), namely, 5-chlorosalicylic acid (5ClSA) and 5-bromosalicylic acid (5BrSA) by sulfate radical (SO4·-) in a thermo-activated persulfate system. The reaction pathways and mechanisms were proposed based on laser flash photolysis (LFP) techniques, HPLC-HRMS and molecular orbital calculations. Our results revealed that efficient removal of 5XSAs could be achieved by thermo-activated persulfate, and phenoxyl radicals were found to play key roles in the primary oxidation pathways. The subsequent transformation of phenoxyl radicals included hydroxylation and coupling processes. The resulting coupling products could undergo secondary reactions with sulfate radical, including dehalogenation, decarboxylation and hydroxylation. Hydroxylated products were in turn oxidized by SO4·-, leading to the ring opening and the formation of a series of small molecular carbonyl byproducts. These processes could be responsible for the mineralization and the release of Br- or Cl-. In addition, the degradation rate constants of 5XSAs increased appreciably with increasing temperature, and higher efficiency of oxidation was observed around neutral initial pH. Moreover, degradation kinetics were found to be hardly affected by dissolved oxygen (DO), showing the possibility of applying SR-AOPs under environmental realistic conditions, not only for surface waters, but also for oxygen-deficient underground waters. The present work could increase our understanding of the reactivity and pathways of halogen phenols widely present in natural waters.
中文翻译:

硫酸根自由基介导的5-卤代水杨酸降解:苯氧基自由基转化途径
在本研究中,我们研究了硫酸根基(SO 4 ·-)对两种5-卤代水杨酸(5XSA),5-氯水杨酸(5ClSA)和5-溴水杨酸(5BrSA)的降解动力学和转化途径。)在热活化过硫酸盐体系中。基于激光闪光光解(LFP)技术,HPLC-HRMS和分子轨道计算,提出了反应途径和机理。我们的结果表明,可以通过热活化过硫酸盐有效去除5XSA,并且发现苯氧基自由基在主要氧化途径中起关键作用。苯氧基自由基的后续转化包括羟基化和偶联过程。所得的偶联产物可能与硫酸根发生二次反应,包括脱卤,脱羧和羟基化。羟基化产物又被SO 4 ·-氧化,导致开环并形成一系列小分子羰基副产物。这些过程可负责矿化和Br释放-或Cl - 。此外,5XSA的降解速率常数随温度升高而明显增加,并且在中性初始pH值附近观察到更高的氧化效率。此外,发现降解动力学几乎不受溶解氧(DO)的影响,这表明在环境现实条件下不仅对地表水,而且对缺氧的地下水应用SR-AOP的可能性。目前的工作可以增加我们对天然水中广泛存在的卤素酚的反应性和途径的了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号