当前位置:
X-MOL 学术
›
Spectrochim. Acta. A Mol. Biomol. Spectrosc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spectroscopy and molecular docking approach for investigation on the binding of nocodazole to human serum albumin.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2020-03-21 , DOI: 10.1016/j.saa.2020.118289 Iqubal Singh 1 , Vijay Luxami 1 , Kamaldeep Paul 1
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2020-03-21 , DOI: 10.1016/j.saa.2020.118289 Iqubal Singh 1 , Vijay Luxami 1 , Kamaldeep Paul 1
Affiliation
|
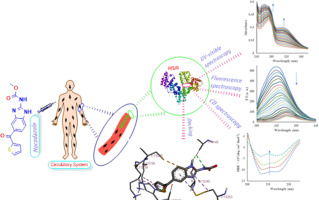
The interaction between nocodazole (Nz) and human serum albumin (HSA) under controlled physiological condition (pH 7.4) is examined using absorption, emission, fluorescence lifetime (FLT) and circular dichroism (CD) spectroscopic techniques. The binding constant (order of 105 M-1) from UV-vis and fluorescence spectroscopy reveals a strong interaction between Nz and HSA. Fluorescence quenching study shows that Nz binds with HSA through static quenching process. It is induced by formation of Nz-HSA complex because the Stern-Volmer quenching constant is inversely correlated with the temperature which is further verified by time-resolved fluorescence spectroscopy. The thermodynamic parameters at different temperatures indicate that the binding process is spontaneous where hydrogen bonding interactions and Van der Waals forces play major roles during the interaction between Nz and HSA. By means of spectroscopy and molecular modeling, we have discovered and interpreted the alteration of the secondary structure of HSA by Nz complexation. Synchronous, three-dimensional fluorescence and CD spectroscopic results reveal that the addition of Nz to HSA affects changes in the micro-environment and conformation of HSA. According to Förster Resonance Energy Transfer (FRET), the binding distance (r) between Nz and residue of HSA is <8 nm with excellent energy efficiency. The docking study suggests that nocodazole binds at Domain IIA in the hydrophobic pocket of human serum albumin.
中文翻译:

光谱学和分子对接方法研究诺考达唑与人血清白蛋白的结合。
使用吸收,发射,荧光寿命(FLT)和圆二色性(CD)光谱技术检查了在受控生理条件(pH 7.4)下诺考达唑(Nz)与人血清白蛋白(HSA)之间的相互作用。UV-vis和荧光光谱的结合常数(105 M-1的数量级)显示Nz和HSA之间有很强的相互作用。荧光猝灭研究表明,Nz通过静态猝灭过程与HSA结合。它是由Nz-HSA配合物的形成引起的,因为Stern-Volmer猝灭常数与温度成反比,而时间分辨荧光光谱进一步证实了这一点。在不同温度下的热力学参数表明,结合过程是自发的,其中氢键相互作用和范德华力在Nz和HSA之间的相互作用中起主要作用。通过光谱学和分子建模,我们已经发现并解释了Nz络合对HSA二级结构的改变。同步的三维荧光和CD光谱结果表明,向HSA添加Nz会影响HSA的微环境和构象的变化。根据福斯特共振能量转移(FRET),Nz与HSA残留物之间的结合距离(r)<8 nm,具有出色的能源效率。对接研究表明,诺考达唑在人血清白蛋白疏水口袋中的IIA域结合。通过光谱学和分子建模,我们已经发现并解释了Nz络合对HSA二级结构的改变。同步的三维荧光和CD光谱结果表明,向HSA添加Nz会影响HSA的微环境和构象的变化。根据福斯特共振能量转移(FRET),Nz与HSA残留物之间的结合距离(r)<8 nm,具有出色的能源效率。对接研究表明,诺考达唑在人血清白蛋白疏水口袋中的IIA域结合。通过光谱学和分子建模,我们已经发现并解释了Nz络合对HSA二级结构的改变。同步的三维荧光和CD光谱结果表明,向HSA添加Nz会影响HSA的微环境和构象的变化。根据福斯特共振能量转移(FRET),Nz与HSA残留物之间的结合距离(r)<8 nm,具有出色的能源效率。对接研究表明,诺考达唑在人血清白蛋白疏水口袋中的IIA域结合。三维荧光和CD光谱结果表明,向HSA添加Nz会影响HSA的微环境和构象的变化。根据福斯特共振能量转移(FRET),Nz与HSA残留物之间的结合距离(r)<8 nm,具有出色的能源效率。对接研究表明,诺考达唑在人血清白蛋白疏水口袋中的IIA域结合。三维荧光和CD光谱结果表明,向HSA添加Nz会影响HSA的微环境和构象的变化。根据福斯特共振能量转移(FRET),Nz与HSA残留物之间的结合距离(r)<8 nm,具有出色的能源效率。对接研究表明,诺考达唑在人血清白蛋白疏水口袋中的IIA域结合。
更新日期:2020-03-22
中文翻译:

光谱学和分子对接方法研究诺考达唑与人血清白蛋白的结合。
使用吸收,发射,荧光寿命(FLT)和圆二色性(CD)光谱技术检查了在受控生理条件(pH 7.4)下诺考达唑(Nz)与人血清白蛋白(HSA)之间的相互作用。UV-vis和荧光光谱的结合常数(105 M-1的数量级)显示Nz和HSA之间有很强的相互作用。荧光猝灭研究表明,Nz通过静态猝灭过程与HSA结合。它是由Nz-HSA配合物的形成引起的,因为Stern-Volmer猝灭常数与温度成反比,而时间分辨荧光光谱进一步证实了这一点。在不同温度下的热力学参数表明,结合过程是自发的,其中氢键相互作用和范德华力在Nz和HSA之间的相互作用中起主要作用。通过光谱学和分子建模,我们已经发现并解释了Nz络合对HSA二级结构的改变。同步的三维荧光和CD光谱结果表明,向HSA添加Nz会影响HSA的微环境和构象的变化。根据福斯特共振能量转移(FRET),Nz与HSA残留物之间的结合距离(r)<8 nm,具有出色的能源效率。对接研究表明,诺考达唑在人血清白蛋白疏水口袋中的IIA域结合。通过光谱学和分子建模,我们已经发现并解释了Nz络合对HSA二级结构的改变。同步的三维荧光和CD光谱结果表明,向HSA添加Nz会影响HSA的微环境和构象的变化。根据福斯特共振能量转移(FRET),Nz与HSA残留物之间的结合距离(r)<8 nm,具有出色的能源效率。对接研究表明,诺考达唑在人血清白蛋白疏水口袋中的IIA域结合。通过光谱学和分子建模,我们已经发现并解释了Nz络合对HSA二级结构的改变。同步的三维荧光和CD光谱结果表明,向HSA添加Nz会影响HSA的微环境和构象的变化。根据福斯特共振能量转移(FRET),Nz与HSA残留物之间的结合距离(r)<8 nm,具有出色的能源效率。对接研究表明,诺考达唑在人血清白蛋白疏水口袋中的IIA域结合。三维荧光和CD光谱结果表明,向HSA添加Nz会影响HSA的微环境和构象的变化。根据福斯特共振能量转移(FRET),Nz与HSA残留物之间的结合距离(r)<8 nm,具有出色的能源效率。对接研究表明,诺考达唑在人血清白蛋白疏水口袋中的IIA域结合。三维荧光和CD光谱结果表明,向HSA添加Nz会影响HSA的微环境和构象的变化。根据福斯特共振能量转移(FRET),Nz与HSA残留物之间的结合距离(r)<8 nm,具有出色的能源效率。对接研究表明,诺考达唑在人血清白蛋白疏水口袋中的IIA域结合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号