当前位置:
X-MOL 学术
›
Acta Biomater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bioactivatable reactive oxygen species-sensitive nanoparticulate system for chemo-photodynamic therapy.
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-03-21 , DOI: 10.1016/j.actbio.2020.03.027 Yugyeong Kim 1 , Saji Uthaman 1 , Shameer Pillarisetti 2 , Kangmin Noh 1 , Kang Moo Huh 1 , In-Kyu Park 2
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-03-21 , DOI: 10.1016/j.actbio.2020.03.027 Yugyeong Kim 1 , Saji Uthaman 1 , Shameer Pillarisetti 2 , Kangmin Noh 1 , Kang Moo Huh 1 , In-Kyu Park 2
Affiliation
|
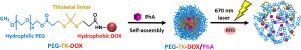
Bioactivatable polymer nanoparticles (NPs) have attracted considerable attention as a prospective cancer therapy. Herein, we describe bioactivatable reactive oxygen species (ROS)-sensitive prodrug NPs designed to elicit spatiotemporally controlled, phototriggered chemo-photodynamic therapy. First, an effective anticancer agent, doxorubicin (DOX), was conjugated to poly(ethylene glycol) (PEG) via an ROS-responsive degradable thioketal (TK) linker. The resulting amphiphilic PEG-DOX conjugate (PEG-TK-DOX) self-assembled into a bioactivatable ROS-responsive NP system could efficiently encapsulate a hydrophobic photodynamic therapy (PDT) agent, pheophorbide A (PhA), with good colloidal stability and unimodal size distribution. Second, after the selective retention of NPs in the tumor, the site-specific release of DOX and PhA was spatiotemporally controlled, initially by endogenous ROS and subsequently by exogenous ROS produced during PDT. The locoregional treatment not only photoactivates PhA molecules to generate cytotoxic ROS but also triggers an ROS cascade, which accelerates the release of DOX and PhA via the ROS-mediated structural destruction of NPs, resulting in an enhanced anticancer therapeutic effect. This prodrug-NP system may function as an effective nanomedicine platform, working synergistically to maximize the efficacy of the combination of chemotherapy and photodynamic therapy with a remote-controlled release mechanism. STATEMENT OF SIGNIFICANCE: Photodynamic therapy (PDT) is a noninvasive therapy involving local ROS generation through the activation of photosensitizer (PS) molecules induced via external irradiation with near-infrared (NIR) light. Combinational therapies with PDT could synergistically enhance the therapeutic efficacy and overcome the limitations of monotherapy. In this study, we describe bioactivatable reactive oxygen species (ROS)-sensitive prodrug nanoparticles designed to elicit spatiotemporally controlled, photo triggered chemo-photodynamic therapy. Upon accumulation in tumor by enhanced permeation and retention (EPR) effect, the nanoparticles exhibited target-specific release of chemo-drug and photosensitizer in a spatiotemporally controlled cascade manner by endogenous ROS in the initial stage and the excessive production of exogenous ROS during PDT, leading to a further ROS cascade that accelerates the release of therapeutic cargo.
中文翻译:

用于化学光动力学治疗的可生物活化的活性氧物种敏感的纳米颗粒系统。
可生物活化的聚合物纳米颗粒(NPs)作为一种潜在的癌症治疗方法已引起了广泛的关注。在这里,我们描述了可生物活化的活性氧(ROS)敏感的前药NPs,旨在引起时空控制的,光触发的化学光动力疗法。首先,将有效的抗癌药阿霉素(DOX)通过ROS响应性可降解硫缩酮(TK)接头与聚(乙二醇)(PEG)偶联。自组装成可生物激活的ROS响应NP系统的两亲性PEG-DOX共轭物(PEG-TK-DOX)可以有效地包裹疏水性光动力疗法(PDT)剂脱镁叶绿酸A(PhA),具有良好的胶体稳定性和单峰尺寸分配。第二,在肿瘤中选择性保留NP之后,DOX和PhA的位点特异性释放是时空控制的,最初是由内源性ROS控制,随后是由PDT过程中产生的外源性ROS控制。局部处理不仅光激活PhA分子以产生细胞毒性ROS,而且触发ROS级联,通过ROS介导的NPs结构破坏,加速DOX和PhA的释放,从而增强抗癌治疗效果。该前药-NP系统可作为有效的纳米药物平台发挥协同作用,以最大限度地发挥化学疗法和光动力疗法与遥控释放机制相结合的功效。重要性声明:光动力疗法(PDT)是一种非侵入性疗法,涉及通过激活光敏剂(PS)分子而产生局部ROS,而光敏剂(PS)分子则是通过用近红外(NIR)光进行外部照射而诱导的。PDT的联合疗法可以协同提高疗效,克服单一疗法的局限性。在这项研究中,我们描述了可生物活化的活性氧(ROS)敏感的前药纳米颗粒,旨在引发时空控制的,光触发的化学光动力疗法。通过增强的渗透和保留(EPR)效应在肿瘤中蓄积后,纳米粒子在初始阶段由内源性ROS时空控制级联方式显示化学药物和光敏剂的靶标特异性释放,而在PDT期间过量产生外源性ROS,
更新日期:2020-03-21
中文翻译:

用于化学光动力学治疗的可生物活化的活性氧物种敏感的纳米颗粒系统。
可生物活化的聚合物纳米颗粒(NPs)作为一种潜在的癌症治疗方法已引起了广泛的关注。在这里,我们描述了可生物活化的活性氧(ROS)敏感的前药NPs,旨在引起时空控制的,光触发的化学光动力疗法。首先,将有效的抗癌药阿霉素(DOX)通过ROS响应性可降解硫缩酮(TK)接头与聚(乙二醇)(PEG)偶联。自组装成可生物激活的ROS响应NP系统的两亲性PEG-DOX共轭物(PEG-TK-DOX)可以有效地包裹疏水性光动力疗法(PDT)剂脱镁叶绿酸A(PhA),具有良好的胶体稳定性和单峰尺寸分配。第二,在肿瘤中选择性保留NP之后,DOX和PhA的位点特异性释放是时空控制的,最初是由内源性ROS控制,随后是由PDT过程中产生的外源性ROS控制。局部处理不仅光激活PhA分子以产生细胞毒性ROS,而且触发ROS级联,通过ROS介导的NPs结构破坏,加速DOX和PhA的释放,从而增强抗癌治疗效果。该前药-NP系统可作为有效的纳米药物平台发挥协同作用,以最大限度地发挥化学疗法和光动力疗法与遥控释放机制相结合的功效。重要性声明:光动力疗法(PDT)是一种非侵入性疗法,涉及通过激活光敏剂(PS)分子而产生局部ROS,而光敏剂(PS)分子则是通过用近红外(NIR)光进行外部照射而诱导的。PDT的联合疗法可以协同提高疗效,克服单一疗法的局限性。在这项研究中,我们描述了可生物活化的活性氧(ROS)敏感的前药纳米颗粒,旨在引发时空控制的,光触发的化学光动力疗法。通过增强的渗透和保留(EPR)效应在肿瘤中蓄积后,纳米粒子在初始阶段由内源性ROS时空控制级联方式显示化学药物和光敏剂的靶标特异性释放,而在PDT期间过量产生外源性ROS,










































 京公网安备 11010802027423号
京公网安备 11010802027423号