当前位置:
X-MOL 学术
›
BBA Bioenerg.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Proton motive function of the terminal antiporter-like subunit in respiratory complex I.
Biochimica et Biophysica Acta (BBA) - Bioenergetics ( IF 3.4 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.bbabio.2020.148185 Amina Djurabekova 1 , Outi Haapanen 1 , Vivek Sharma 2
Biochimica et Biophysica Acta (BBA) - Bioenergetics ( IF 3.4 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.bbabio.2020.148185 Amina Djurabekova 1 , Outi Haapanen 1 , Vivek Sharma 2
Affiliation
|
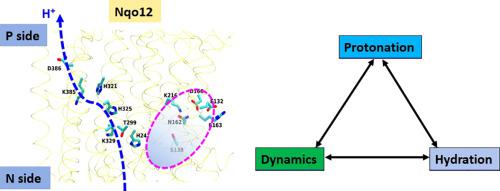
In the aerobic respiratory chains of many organisms, complex I functions as the first electron input. By reducing ubiquinone (Q) to ubiquinol, it catalyzes the translocation of protons across the membrane as far as ~200 Å from the site of redox reactions. Despite significant amount of structural and biochemical data, the details of redox coupled proton pumping in complex I are poorly understood. In particular, the proton transfer pathways are extremely difficult to characterize with the current structural and biochemical techniques. Here, we applied multiscale computational approaches to identify the proton transfer paths in the terminal antiporter-like subunit of complex I. Data from combined classical and quantum chemical simulations reveal for the first time structural elements that are exclusive to the subunit, and enables the enzyme to achieve coupling between the spatially separated Q redox reactions and proton pumping. By studying long time scale protonation and hydration dependent conformational dynamics of key amino acid residues, we provide novel insights into the proton pumping mechanism of complex I.
中文翻译:

呼吸复合体I中末端反向转运蛋白样亚基的质子运动功能。
在许多生物体的有氧呼吸链中,复合物I充当第一个电子输入。通过将泛醌(Q)还原为泛醇,它催化质子从氧化还原反应位点到整个膜的转移距离约为200Å。尽管有大量的结构和生化数据,但对复合物I中氧化还原偶联质子泵浦的细节知之甚少。特别地,用当前的结构和生化技术很难表征质子转移途径。在这里,我们应用了多尺度计算方法来识别复合物I末端类似反向转运子的亚单元中的质子传递路径。经典和量子化学模拟相结合的数据首次揭示了该亚单元专有的结构元素,并使酶能够在空间上分离的Q氧化还原反应和质子泵浦之间实现偶联。通过研究关键氨基酸残基的长时间尺度质子化和水合依赖性构象动力学,我们提供了对复杂I的质子泵浦机制的新见解。
更新日期:2020-04-20
中文翻译:

呼吸复合体I中末端反向转运蛋白样亚基的质子运动功能。
在许多生物体的有氧呼吸链中,复合物I充当第一个电子输入。通过将泛醌(Q)还原为泛醇,它催化质子从氧化还原反应位点到整个膜的转移距离约为200Å。尽管有大量的结构和生化数据,但对复合物I中氧化还原偶联质子泵浦的细节知之甚少。特别地,用当前的结构和生化技术很难表征质子转移途径。在这里,我们应用了多尺度计算方法来识别复合物I末端类似反向转运子的亚单元中的质子传递路径。经典和量子化学模拟相结合的数据首次揭示了该亚单元专有的结构元素,并使酶能够在空间上分离的Q氧化还原反应和质子泵浦之间实现偶联。通过研究关键氨基酸残基的长时间尺度质子化和水合依赖性构象动力学,我们提供了对复杂I的质子泵浦机制的新见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号