iScience ( IF 4.6 ) Pub Date : 2020-03-21 , DOI: 10.1016/j.isci.2020.101001 Xiaoyan Zhuang 1 , Aihui Zhang 1 , Siyao Qiu 2 , Chun Tang 3 , Shiqiang Zhao 3 , Hongchun Li 4 , Yonghui Zhang 5 , Yali Wang 1 , Binju Wang 3 , Baishan Fang 1 , Wenjing Hong 3
|
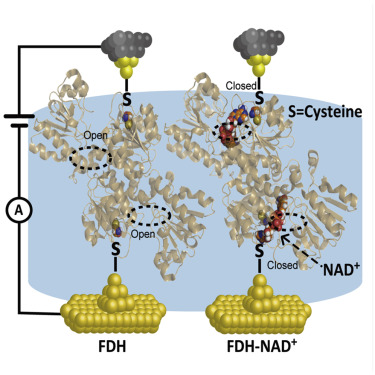
Oxidation of formate to CO2 is catalyzed via the donation of electrons from formate dehydrogenase (FDH) to nicotinamide adenine dinucleotide (NAD+), and thus the charge transport characteristics of FDH become essential but remain unexplored. Here, we investigated the charge transport through single-enzyme junctions of FDH using the scanning tunneling microscope break junction technique (STM-BJ). We found that the coupling of NAD+ with FDH boosts the charge transport by ∼2,100%, and the single-enzyme conductance highly correlates with the enzyme activity. The combined flicker noise analysis demonstrated the switching of the coenzyme-mediated charge transport pathway and supported by the significantly reduced HOMO-LUMO gap from calculations. Site-specific mutagenesis analysis demonstrated that FDH-NAD+ stably combined own higher bioactivity and boosts charge transport, and the coupling has been optimized via the natural selection. Our work provides evidence of hydrogen bond coupling in bioactivity but also bridges the charge transport through single-enzyme junctions and enzyme activities.
中文翻译:

辅酶偶联通过单个生物活性酶连接促进电荷传输。
通过将电子从甲酸脱氢酶(FDH)赠予烟酰胺腺嘌呤二核苷酸(NAD +)催化将甲酸氧化为CO 2,因此FDH的电荷传输特性变得必不可少,但仍未开发。在这里,我们使用扫描隧道显微镜断裂连接技术(STM-BJ)研究了通过FDH单酶连接的电荷传输。我们发现NAD +的耦合使用FDH时,电荷输送增加了约2,100%,并且单酶电导率与酶活性高度相关。组合的闪烁噪声分析证明了辅酶介导的电荷传输途径的切换,并得到了计算显着降低的HOMO-LUMO缺口的支持。特定于位点的诱变分析表明,FDH-NAD +稳定地结合在一起具有更高的生物活性并增强了电荷传输,并且通过自然选择对偶联进行了优化。我们的工作提供了生物活性中氢键偶联的证据,但也通过单酶连接和酶活性架起了电荷转移的桥梁。











































 京公网安备 11010802027423号
京公网安备 11010802027423号