当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Axially Chiral Aryl‐Alkene‐Indole Framework: A Nascent Member of the Atropisomeric Family and Its Catalytic Asymmetric Construction
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-04-04 , DOI: 10.1002/cjoc.202000131 Cong‐Shuai Wang 1 , Tian‐Zhen Li 1 , Si‐Jia Liu 1 , Yu‐Chen Zhang 1 , Shuang Deng 2 , Yinchun Jiao 2 , Feng Shi 1
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-04-04 , DOI: 10.1002/cjoc.202000131 Cong‐Shuai Wang 1 , Tian‐Zhen Li 1 , Si‐Jia Liu 1 , Yu‐Chen Zhang 1 , Shuang Deng 2 , Yinchun Jiao 2 , Feng Shi 1
Affiliation
|
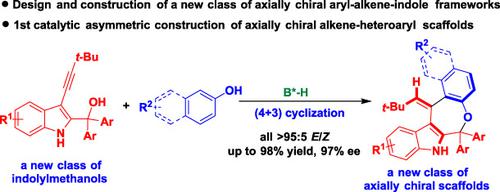
A new class of axially chiral aryl‐alkene‐indole frameworks have been designed, and the first catalytic asymmetric construction of such scaffolds has been established by the strategy of organocatalytic (Z/E)‐selective and enantioselective (4+3) cyclization of 3‐alkynyl‐2‐indolylmethanols with 2‐naphthols or phenols (all >95 : 5 E/Z, up to 98% yield, 97% ee). This reaction also represents the first catalytic asymmetric construction of axially chiral alkene‐heteroaryl scaffolds, which will add a new member to the atropisomeric family. This approach has not only confronted the great challenges in constructing axially chiral alkene‐heteroaryl scaffolds but also provided a powerful strategy for the enantioselective construction of axially chiral aryl‐alkene‐indole frameworks.
中文翻译:

轴向手性芳基-烯烃-吲哚骨架:阻转异构家族的新生成员及其催化不对称结构
设计了一类新的轴向手性芳基-烯烃-吲哚骨架,并通过3的有机催化(Z / E)选择性和对映选择性(4 + 3)环化策略建立了此类支架的第一个催化不对称结构。带有2-萘酚或苯酚的炔基-2-吲哚基甲醇(均> 95:5 E / Z,产率高达98%,ee高达97%)。该反应也代表了轴向手性烯烃-杂芳基骨架的第一个催化不对称结构,它将为阻转异构体家族增加一个新成员。这种方法不仅在构建轴向手性烯烃-杂芳基骨架方面面临巨大挑战,而且为轴向手性芳基烯烃-吲哚骨架的对映选择性构建提供了强有力的策略。
更新日期:2020-04-04
中文翻译:

轴向手性芳基-烯烃-吲哚骨架:阻转异构家族的新生成员及其催化不对称结构
设计了一类新的轴向手性芳基-烯烃-吲哚骨架,并通过3的有机催化(Z / E)选择性和对映选择性(4 + 3)环化策略建立了此类支架的第一个催化不对称结构。带有2-萘酚或苯酚的炔基-2-吲哚基甲醇(均> 95:5 E / Z,产率高达98%,ee高达97%)。该反应也代表了轴向手性烯烃-杂芳基骨架的第一个催化不对称结构,它将为阻转异构体家族增加一个新成员。这种方法不仅在构建轴向手性烯烃-杂芳基骨架方面面临巨大挑战,而且为轴向手性芳基烯烃-吲哚骨架的对映选择性构建提供了强有力的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号