Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regulation of Leishmania major PAS domain‐containing phosphoglycerate kinase by cofactor Mg2+ ion at neutral pH
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-03-20 , DOI: 10.1111/febs.15305 Saroj Biswas 1 , Ayan Adhikari 1 , Aditi Mukherjee 1 , Sumit Das 1 , Subrata Adak 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-03-20 , DOI: 10.1111/febs.15305 Saroj Biswas 1 , Ayan Adhikari 1 , Aditi Mukherjee 1 , Sumit Das 1 , Subrata Adak 1
Affiliation
|
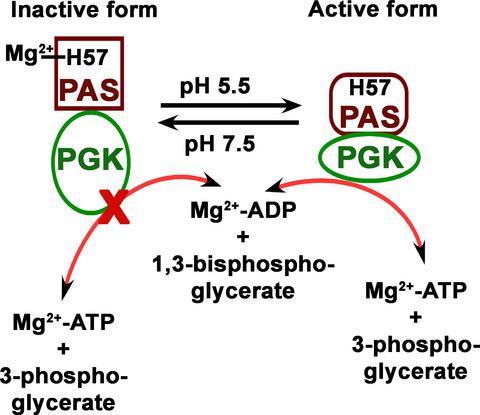
Recently, we described the PAS domain‐containing phosphoglycerate kinase (PGK) from Leishmania major (LmPAS‐PGK) that shows acidic pH (5.5)‐dependent optimum catalytic activity. The PAS domain of LmPAS‐PGK is expected to regulate PGK activity during catalysis, but the mechanism of regulation by PAS domain at the molecular level is uncharacterized. In this work, we have utilized the full‐length, PAS domain‐deleted, and mutant enzymes to measure the enzymatic activity in the presence of divalent cation at various pH values. Catalytic activity measurement indicates that Mg2+ binding through PAS domain inhibits the PGK activity at pH 7.5, and this inhibition is withdrawn at pH 5.5. To identify the Mg2+ binding residues of the PAS domain, we exploited a systematic mutational analysis of all (four) His residues in the PAS domain for potential divalent cation binding. Replacement of His‐57 with alanine resulted in depression in the presence of Mg2+ at pH 7.5, but H71A, H89A, and H111A showed similar characteristics with respect to the wild‐type protein. Fluorescence and isothermal titration calorimetry studies revealed that H57 is responsible for Mg2+ binding in the absence of substrates. Thus, the protonated form of His57 at acidic pH 5.5 destabilizes the Mg2+ binding in the PAS domain, which is an essential requirement in the wild‐type LmPAS‐PGK for a conformational alteration in the sensor domain that, sequentially, activates the PGK domain, resulting in the synthesis of higher amounts of ATP.
中文翻译:

辅助因子Mg2 +离子在中性pH值下调节利什曼原虫含有PAS结构域的磷酸甘油酸激酶
最近,我们描述了来自利什曼原虫(LmPAS-PGK)的含有PAS域的磷酸甘油酸激酶(PGK),它显示了酸性pH(5.5)依赖性的最佳催化活性。LmPAS-PGK的PAS结构域有望在催化过程中调节PGK活性,但是在分子水平上由PAS结构域调节的机制尚不清楚。在这项工作中,我们利用全长,PAS结构域缺失的突变酶来测量在各种pH值下存在二价阳离子时的酶活性。催化活性的测量表明,通过PAS结构域的Mg 2+结合在pH 7.5时抑制了PGK活性,并且在pH 5.5时取消了该抑制作用。识别镁2+结合PAS域的残基,我们对PAS域中所有(四个)His残基进行了系统的突变分析,以发现可能的二价阳离子结合。在pH值为7.5的Mg 2+存在下,用丙氨酸替代His-57导致抑郁症,但是H71A,H89A和H111A与野生型蛋白表现出相似的特征。荧光和等温滴定量热法研究表明,在没有底物的情况下,H57负责Mg 2+的结合。因此,在酸性pH 5.5下,His57的质子化形式会使Mg 2+不稳定 在PAS结构域中进行结合,这是野生型LmPAS-PGK对传感器结构域构象改变的基本要求,该构象改变会依次激活PGK结构域,从而导致合成更高含量的ATP。
更新日期:2020-03-20
中文翻译:

辅助因子Mg2 +离子在中性pH值下调节利什曼原虫含有PAS结构域的磷酸甘油酸激酶
最近,我们描述了来自利什曼原虫(LmPAS-PGK)的含有PAS域的磷酸甘油酸激酶(PGK),它显示了酸性pH(5.5)依赖性的最佳催化活性。LmPAS-PGK的PAS结构域有望在催化过程中调节PGK活性,但是在分子水平上由PAS结构域调节的机制尚不清楚。在这项工作中,我们利用全长,PAS结构域缺失的突变酶来测量在各种pH值下存在二价阳离子时的酶活性。催化活性的测量表明,通过PAS结构域的Mg 2+结合在pH 7.5时抑制了PGK活性,并且在pH 5.5时取消了该抑制作用。识别镁2+结合PAS域的残基,我们对PAS域中所有(四个)His残基进行了系统的突变分析,以发现可能的二价阳离子结合。在pH值为7.5的Mg 2+存在下,用丙氨酸替代His-57导致抑郁症,但是H71A,H89A和H111A与野生型蛋白表现出相似的特征。荧光和等温滴定量热法研究表明,在没有底物的情况下,H57负责Mg 2+的结合。因此,在酸性pH 5.5下,His57的质子化形式会使Mg 2+不稳定 在PAS结构域中进行结合,这是野生型LmPAS-PGK对传感器结构域构象改变的基本要求,该构象改变会依次激活PGK结构域,从而导致合成更高含量的ATP。









































 京公网安备 11010802027423号
京公网安备 11010802027423号