当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nitrogen and Sulfur Vacancies in Carbon Shell to Tune Charge Distribution of Co6Ni3S8 Core and Boost Sodium Storage
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2020-03-20 , DOI: 10.1002/aenm.201904147 Yihui Zou 1 , Yu Gu 1 , Bin Hui 1 , Xianfeng Yang 2 , Hongwei Liu 3 , Shuai Chen 4 , Rongsheng Cai 5 , Jin Sun 1 , Xiaoli Zhang 6 , Dongjiang Yang 1, 7
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2020-03-20 , DOI: 10.1002/aenm.201904147 Yihui Zou 1 , Yu Gu 1 , Bin Hui 1 , Xianfeng Yang 2 , Hongwei Liu 3 , Shuai Chen 4 , Rongsheng Cai 5 , Jin Sun 1 , Xiaoli Zhang 6 , Dongjiang Yang 1, 7
Affiliation
|
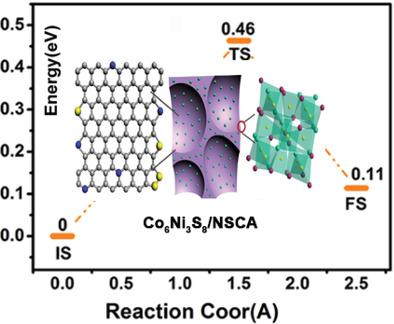
Recently, the metal sulfide‐carbon nanocomposites have been suggested as a low‐cost alternative to lithium ion batteries, but commercial application is seriously hindered by their relatively inferior cyclic performance. Herein, N and S vacancies in an N,S co‐doped carbon (NSC) shell for anchoring a new bimetallic sulfide core of Co6Ni3S8 using Co‐Ni‐alginate biomass are introduced. The obtained Co6Ni3S8/carbon aerogels (Co6Ni3S8/NSCA) exhibit excellent sodium‐ion storage properties, high reversible capacity (568.1 mAh g−1 at 1 A g−1), and an excellent cycle stability (94.4% after 300 cycles). Density functional theory calculation results disclose that nitrogen and sulfur vacancies in the carbon shell can enhance the binding between the Co6Ni3S8 core and NSC shell, ensuring an improved structural and electrochemical stability. In addition, an increased adsorption energy of Na+ (−1.88 eV) and a decreased barrier energy for Na+ diffusion (0.46 eV) are observed indicating a fast Na+ diffusion process. The powder X‐ray diffraction refinement confirms that the lattice parameters of Co6Ni3S8 extend to 0.9972 nm compared with Co9S8 (0.9928 nm), suppressing the volume expansion in Na+ diffusion processes.
中文翻译:

碳壳中的氮和硫空位可调节Co6Ni3S8核的电荷分布并增强钠存储
最近,有人提出将金属硫化物-碳纳米复合材料作为锂离子电池的低成本替代品,但由于其相对较差的循环性能,严重阻碍了其商业应用。本文介绍了N,S共掺杂碳(NSC)壳中的N和S空位,用于利用Co-Ni-藻酸盐生物质锚定Co 6 Ni 3 S 8的新双金属硫化物核。使得到的共6的Ni 3小号8 /碳气凝胶(联合6的Ni 3小号8 / NSCA)表现出优异的钠离子存储性能,高可逆容量(毫安568.1克-1 1 A G -1)和出色的循环稳定性(300次循环后为94.4%)。密度泛函理论计算结果表明,碳壳中的氮和硫空位可以增强Co 6 Ni 3 S 8核与NSC壳之间的结合,从而确保改善的结构和电化学稳定性。另外,观察到Na +的吸附能增加(-1.88 eV),Na +扩散的势垒能降低(0.46 eV),这表明Na +的扩散过程很快。粉末X射线衍射细化证实,与Co相比,Co 6 Ni 3 S 8的晶格参数扩展到0.9972 nm9 S 8(0.9928 nm),抑制了Na +扩散过程中的体积膨胀。
更新日期:2020-03-20
中文翻译:

碳壳中的氮和硫空位可调节Co6Ni3S8核的电荷分布并增强钠存储
最近,有人提出将金属硫化物-碳纳米复合材料作为锂离子电池的低成本替代品,但由于其相对较差的循环性能,严重阻碍了其商业应用。本文介绍了N,S共掺杂碳(NSC)壳中的N和S空位,用于利用Co-Ni-藻酸盐生物质锚定Co 6 Ni 3 S 8的新双金属硫化物核。使得到的共6的Ni 3小号8 /碳气凝胶(联合6的Ni 3小号8 / NSCA)表现出优异的钠离子存储性能,高可逆容量(毫安568.1克-1 1 A G -1)和出色的循环稳定性(300次循环后为94.4%)。密度泛函理论计算结果表明,碳壳中的氮和硫空位可以增强Co 6 Ni 3 S 8核与NSC壳之间的结合,从而确保改善的结构和电化学稳定性。另外,观察到Na +的吸附能增加(-1.88 eV),Na +扩散的势垒能降低(0.46 eV),这表明Na +的扩散过程很快。粉末X射线衍射细化证实,与Co相比,Co 6 Ni 3 S 8的晶格参数扩展到0.9972 nm9 S 8(0.9928 nm),抑制了Na +扩散过程中的体积膨胀。











































 京公网安备 11010802027423号
京公网安备 11010802027423号