当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Perfluoroolefin complexes versus perfluorometallacycles and perfluorocarbene complexes in cyclopentadienylcobalt chemistry
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020/03/20 , DOI: 10.1039/c9cp06685c Limei Wen 1, 2, 3, 4, 5 , Guoliang Li 1, 2, 3, 4, 5 , Yaoming Xie 6, 7, 8, 9 , R. Bruce King 6, 7, 8, 9 , Henry F. Schaefer 6, 7, 8, 9
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020/03/20 , DOI: 10.1039/c9cp06685c Limei Wen 1, 2, 3, 4, 5 , Guoliang Li 1, 2, 3, 4, 5 , Yaoming Xie 6, 7, 8, 9 , R. Bruce King 6, 7, 8, 9 , Henry F. Schaefer 6, 7, 8, 9
Affiliation
|
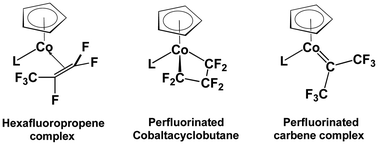
Fluorocarbons have been shown experimentally by Baker and coworkers to combine with the cyclopentadienylcobalt (CpCo) moiety to form fluoroolefin and fluorocarbene complexes as well as fluorinated cobaltacyclic rings. In this connection density functional theory (DFT) studies on the cyclopentadienylcobalt fluorocarbon complexes CpCo(L)(CnF2n) (L = CO, PMe3; n = 3 and 4) indicate structures with perfluoroolefin ligands to be the lowest energy structures followed by perfluorometallacycle structures and finally by structures with perfluorocarbene ligands. Thus, for the CpCo(L)(C3F6) (L = CO, PMe3) complexes, the perfluoropropene structure has the lowest energy, followed by the perfluorocobaltacyclobutane structure and the perfluoroisopropylidene structure less stable by 8 to 11 kcal mol−1, and the highest energy perfluoropropylidene structure less stable by more than 12 kcal mol−1. For the two metal carbene structures Cp(L)Co![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CF3)2 and Cp(L)Co
C(CF3)2 and Cp(L)Co![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF(C2F5), the former is more stable than the latter, even though the latter has Fischer carbene character. For the CpCo(L)(C4F8) (L = CO, PMe3) complexes, the perfluoroolefin complex structures have the lowest energies, followed by the perfluorometallacycle structures at 10 to 20 kcal mol−1, and the structures with perfluorocarbene ligands at yet higher energies more than 20 kcal mol−1 above the lowest energy structure. This is consistent with the experimentally observed isomerization of the perfluorinated cobaltacyclobutane complexes CpCo(PPh2Me)(–CFR–CF2–CF2–) (R = F, CF3) to the perfluoroolefin complexes CpCo(PPh2Me)(RCF
CF(C2F5), the former is more stable than the latter, even though the latter has Fischer carbene character. For the CpCo(L)(C4F8) (L = CO, PMe3) complexes, the perfluoroolefin complex structures have the lowest energies, followed by the perfluorometallacycle structures at 10 to 20 kcal mol−1, and the structures with perfluorocarbene ligands at yet higher energies more than 20 kcal mol−1 above the lowest energy structure. This is consistent with the experimentally observed isomerization of the perfluorinated cobaltacyclobutane complexes CpCo(PPh2Me)(–CFR–CF2–CF2–) (R = F, CF3) to the perfluoroolefin complexes CpCo(PPh2Me)(RCF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF2) in the presence of catalytic quantities of HN(SO2CF3)2. Further refinement of the relative energies by the state-of-the-art DLPNO-CCSD(T) method gives results essentially consistent with the DFT results summarized above.
CF2) in the presence of catalytic quantities of HN(SO2CF3)2. Further refinement of the relative energies by the state-of-the-art DLPNO-CCSD(T) method gives results essentially consistent with the DFT results summarized above.
中文翻译:

环戊二烯钴化学中的全氟烯烃配合物与全氟金属环氧化物和全氟卡宾配合物
贝克和他的同事已经通过实验证明了碳氟化合物与环戊二烯基钴(CpCo)部分结合形成氟烯烃和氟碳烯配合物以及氟化钴环。在这方面,对环戊二烯基钴碳氟化合物配合物CpCo(L)(C n F 2 n)(L = CO,PMe 3 ; n = 3和4)的密度泛函理论(DFT)研究表明,具有全氟烯烃配体的结构具有最低的能量结构,然后是全氟金属环结构,最后是具有全氟卡宾配体的结构。因此,对于CpCo(L)(C 3 F 6)(L = CO,PMe 3)配合物时,全氟丙烯结构的能量最低,其次是全氟钴环丁烷结构和全氟异亚丙基结构,稳定性低8至11 kcal mol -1,最高能量全氟亚丙基结构不稳定,其稳定性高至12 kcal mol -1以上。对于两个金属卡宾结构Cp(L)Co![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) C(CF 3)2和Cp(L)Co
C(CF 3)2和Cp(L)Co ![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) CF(C 2 F 5),前者比后者更稳定,即使后者具有Fischer卡宾特性。对于CpCo(L)(C 4 F 8)(L = CO,PMe 3)配合物,全氟烯烃配合物结构的能量最低,其次是全氟金属环氧化物结构的10至20 kcal mol -1,具有全氟卡宾配体的结构的能量仍比最低能量结构高出20 kcal mol -1。这与通过实验观察到的全氟化钴环丁烷配合物CpCo(PPh 2 Me)(– CFR–CF 2 –CF 2 –)(R = F,CF 3)异构化为全氟烯烃配合物CpCo(PPh 2 Me)(RCF)
CF(C 2 F 5),前者比后者更稳定,即使后者具有Fischer卡宾特性。对于CpCo(L)(C 4 F 8)(L = CO,PMe 3)配合物,全氟烯烃配合物结构的能量最低,其次是全氟金属环氧化物结构的10至20 kcal mol -1,具有全氟卡宾配体的结构的能量仍比最低能量结构高出20 kcal mol -1。这与通过实验观察到的全氟化钴环丁烷配合物CpCo(PPh 2 Me)(– CFR–CF 2 –CF 2 –)(R = F,CF 3)异构化为全氟烯烃配合物CpCo(PPh 2 Me)(RCF)![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) CF 2)在HN(SO的催化量存在2 CF 3)2。通过最新的DLPNO-CCSD(T)方法对相对能量进行进一步细化,得出的结果与上述DFT结果基本一致。
CF 2)在HN(SO的催化量存在2 CF 3)2。通过最新的DLPNO-CCSD(T)方法对相对能量进行进一步细化,得出的结果与上述DFT结果基本一致。
更新日期:2020-04-08
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CF3)2 and Cp(L)Co
C(CF3)2 and Cp(L)Co![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF(C2F5), the former is more stable than the latter, even though the latter has Fischer carbene character. For the CpCo(L)(C4F8) (L = CO, PMe3) complexes, the perfluoroolefin complex structures have the lowest energies, followed by the perfluorometallacycle structures at 10 to 20 kcal mol−1, and the structures with perfluorocarbene ligands at yet higher energies more than 20 kcal mol−1 above the lowest energy structure. This is consistent with the experimentally observed isomerization of the perfluorinated cobaltacyclobutane complexes CpCo(PPh2Me)(–CFR–CF2–CF2–) (R = F, CF3) to the perfluoroolefin complexes CpCo(PPh2Me)(RCF
CF(C2F5), the former is more stable than the latter, even though the latter has Fischer carbene character. For the CpCo(L)(C4F8) (L = CO, PMe3) complexes, the perfluoroolefin complex structures have the lowest energies, followed by the perfluorometallacycle structures at 10 to 20 kcal mol−1, and the structures with perfluorocarbene ligands at yet higher energies more than 20 kcal mol−1 above the lowest energy structure. This is consistent with the experimentally observed isomerization of the perfluorinated cobaltacyclobutane complexes CpCo(PPh2Me)(–CFR–CF2–CF2–) (R = F, CF3) to the perfluoroolefin complexes CpCo(PPh2Me)(RCF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF2) in the presence of catalytic quantities of HN(SO2CF3)2. Further refinement of the relative energies by the state-of-the-art DLPNO-CCSD(T) method gives results essentially consistent with the DFT results summarized above.
CF2) in the presence of catalytic quantities of HN(SO2CF3)2. Further refinement of the relative energies by the state-of-the-art DLPNO-CCSD(T) method gives results essentially consistent with the DFT results summarized above.
中文翻译:

环戊二烯钴化学中的全氟烯烃配合物与全氟金属环氧化物和全氟卡宾配合物
贝克和他的同事已经通过实验证明了碳氟化合物与环戊二烯基钴(CpCo)部分结合形成氟烯烃和氟碳烯配合物以及氟化钴环。在这方面,对环戊二烯基钴碳氟化合物配合物CpCo(L)(C n F 2 n)(L = CO,PMe 3 ; n = 3和4)的密度泛函理论(DFT)研究表明,具有全氟烯烃配体的结构具有最低的能量结构,然后是全氟金属环结构,最后是具有全氟卡宾配体的结构。因此,对于CpCo(L)(C 3 F 6)(L = CO,PMe 3)配合物时,全氟丙烯结构的能量最低,其次是全氟钴环丁烷结构和全氟异亚丙基结构,稳定性低8至11 kcal mol -1,最高能量全氟亚丙基结构不稳定,其稳定性高至12 kcal mol -1以上。对于两个金属卡宾结构Cp(L)Co
![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) C(CF 3)2和Cp(L)Co
C(CF 3)2和Cp(L)Co ![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) CF(C 2 F 5),前者比后者更稳定,即使后者具有Fischer卡宾特性。对于CpCo(L)(C 4 F 8)(L = CO,PMe 3)配合物,全氟烯烃配合物结构的能量最低,其次是全氟金属环氧化物结构的10至20 kcal mol -1,具有全氟卡宾配体的结构的能量仍比最低能量结构高出20 kcal mol -1。这与通过实验观察到的全氟化钴环丁烷配合物CpCo(PPh 2 Me)(– CFR–CF 2 –CF 2 –)(R = F,CF 3)异构化为全氟烯烃配合物CpCo(PPh 2 Me)(RCF)
CF(C 2 F 5),前者比后者更稳定,即使后者具有Fischer卡宾特性。对于CpCo(L)(C 4 F 8)(L = CO,PMe 3)配合物,全氟烯烃配合物结构的能量最低,其次是全氟金属环氧化物结构的10至20 kcal mol -1,具有全氟卡宾配体的结构的能量仍比最低能量结构高出20 kcal mol -1。这与通过实验观察到的全氟化钴环丁烷配合物CpCo(PPh 2 Me)(– CFR–CF 2 –CF 2 –)(R = F,CF 3)异构化为全氟烯烃配合物CpCo(PPh 2 Me)(RCF)![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) CF 2)在HN(SO的催化量存在2 CF 3)2。通过最新的DLPNO-CCSD(T)方法对相对能量进行进一步细化,得出的结果与上述DFT结果基本一致。
CF 2)在HN(SO的催化量存在2 CF 3)2。通过最新的DLPNO-CCSD(T)方法对相对能量进行进一步细化,得出的结果与上述DFT结果基本一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号