当前位置:
X-MOL 学术
›
PLOS Negl. Trop. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intracellular DNA replication and differentiation of Trypanosoma cruzi is asynchronous within individual host cells in vivo at all stages of infection.
PLOS Neglected Tropical Diseases ( IF 3.4 ) Pub Date : 2020-03-20 , DOI: 10.1371/journal.pntd.0008007 Martin C Taylor 1 , Alexander Ward 1 , Francisco Olmo 1 , Shiromani Jayawardhana 1 , Amanda F Francisco 1 , Michael D Lewis 1 , John M Kelly 1
PLOS Neglected Tropical Diseases ( IF 3.4 ) Pub Date : 2020-03-20 , DOI: 10.1371/journal.pntd.0008007 Martin C Taylor 1 , Alexander Ward 1 , Francisco Olmo 1 , Shiromani Jayawardhana 1 , Amanda F Francisco 1 , Michael D Lewis 1 , John M Kelly 1
Affiliation
|
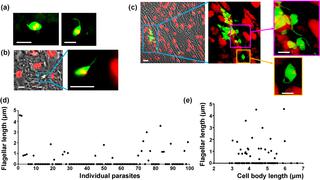
Investigations into intracellular replication and differentiation of Trypanosoma cruzi within the mammalian host have been restricted by limitations in our ability to detect parasitized cells throughout the course of infection. We have overcome this problem by generating genetically modified parasites that express a bioluminescent/fluorescent fusion protein. By combining in vivo imaging and confocal microscopy, this has enabled us to routinely visualise murine infections at the level of individual host cells. These studies reveal that intracellular parasite replication is an asynchronous process, irrespective of tissue location or disease stage. Furthermore, using TUNEL assays and EdU labelling, we demonstrate that within individual infected cells, replication of both mitochondrial (kDNA) and nuclear genomes is not co-ordinated within the parasite population, and that replicating amastigotes and non-replicating trypomastigotes can co-exist in the same cell. Finally, we report the presence of distinct non-canonical morphological forms of T. cruzi in the mammalian host. These appear to represent transitional forms in the amastigote to trypomastigote differentiation process. Therefore, the intracellular life-cycle of T. cruzi in vivo is more complex than previously realised, with potential implications for our understanding of disease pathogenesis, immune evasion and drug development. Dissecting the mechanisms involved will be an important experimental challenge.
中文翻译:

克鲁氏锥虫的细胞内DNA复制和分化在感染的各个阶段在体内单个宿主细胞内都是异步的。
哺乳动物宿主内克氏锥虫细胞内复制和分化的研究受到我们在整个感染过程中检测寄生虫细胞能力的限制。我们已经通过产生表达生物发光/荧光融合蛋白的基因修饰的寄生虫来克服了这个问题。通过结合体内成像和共聚焦显微镜,这使我们能够常规地观察单个宿主细胞水平上的鼠类感染。这些研究表明,细胞内寄生虫复制是一个异步过程,与组织位置或疾病阶段无关。此外,通过TUNEL分析和EdU标记,我们证明了在单个感染的细胞内,线粒体(kDNA)和核基因组的复制在寄生虫种群中并不协调,复制的变形虫和非复制的锥虫病可以在同一细胞中共存。最后,我们报告了哺乳动物宿主中特异克鲁氏梭菌的不同非典型形态学形式的存在。这些似乎代表了从吻合后到锥po的分化过程中的过渡形式。因此,体内克鲁氏锥虫的细胞内生命周期比以前实现的更为复杂,对我们对疾病的发病机理,免疫逃逸和药物开发的理解具有潜在的意义。剖析所涉及的机制将是一项重要的实验挑战。我们报告了在哺乳动物宿主中不同的非规范的克鲁氏梭菌形态学形式的存在。这些似乎代表了从吻合后到锥po的分化过程中的过渡形式。因此,体内克鲁氏锥虫的细胞内生命周期比以前实现的更为复杂,对我们对疾病的发病机理,免疫逃逸和药物开发的理解具有潜在的意义。剖析所涉及的机制将是一项重要的实验挑战。我们报告了在哺乳动物宿主中不同的非规范的克鲁氏梭菌形态学形式的存在。这些似乎代表了从吻合后到锥po的分化过程中的过渡形式。因此,体内克鲁氏锥虫的细胞内生命周期比以前实现的更为复杂,对我们对疾病的发病机理,免疫逃逸和药物开发的理解具有潜在的意义。剖析所涉及的机制将是一项重要的实验挑战。对于我们对疾病的发病机理,免疫逃逸和药物开发的理解具有潜在的意义。剖析所涉及的机制将是一项重要的实验挑战。对于我们对疾病的发病机理,免疫逃逸和药物开发的理解具有潜在的意义。剖析所涉及的机制将是一项重要的实验挑战。
更新日期:2020-03-21
中文翻译:

克鲁氏锥虫的细胞内DNA复制和分化在感染的各个阶段在体内单个宿主细胞内都是异步的。
哺乳动物宿主内克氏锥虫细胞内复制和分化的研究受到我们在整个感染过程中检测寄生虫细胞能力的限制。我们已经通过产生表达生物发光/荧光融合蛋白的基因修饰的寄生虫来克服了这个问题。通过结合体内成像和共聚焦显微镜,这使我们能够常规地观察单个宿主细胞水平上的鼠类感染。这些研究表明,细胞内寄生虫复制是一个异步过程,与组织位置或疾病阶段无关。此外,通过TUNEL分析和EdU标记,我们证明了在单个感染的细胞内,线粒体(kDNA)和核基因组的复制在寄生虫种群中并不协调,复制的变形虫和非复制的锥虫病可以在同一细胞中共存。最后,我们报告了哺乳动物宿主中特异克鲁氏梭菌的不同非典型形态学形式的存在。这些似乎代表了从吻合后到锥po的分化过程中的过渡形式。因此,体内克鲁氏锥虫的细胞内生命周期比以前实现的更为复杂,对我们对疾病的发病机理,免疫逃逸和药物开发的理解具有潜在的意义。剖析所涉及的机制将是一项重要的实验挑战。我们报告了在哺乳动物宿主中不同的非规范的克鲁氏梭菌形态学形式的存在。这些似乎代表了从吻合后到锥po的分化过程中的过渡形式。因此,体内克鲁氏锥虫的细胞内生命周期比以前实现的更为复杂,对我们对疾病的发病机理,免疫逃逸和药物开发的理解具有潜在的意义。剖析所涉及的机制将是一项重要的实验挑战。我们报告了在哺乳动物宿主中不同的非规范的克鲁氏梭菌形态学形式的存在。这些似乎代表了从吻合后到锥po的分化过程中的过渡形式。因此,体内克鲁氏锥虫的细胞内生命周期比以前实现的更为复杂,对我们对疾病的发病机理,免疫逃逸和药物开发的理解具有潜在的意义。剖析所涉及的机制将是一项重要的实验挑战。对于我们对疾病的发病机理,免疫逃逸和药物开发的理解具有潜在的意义。剖析所涉及的机制将是一项重要的实验挑战。对于我们对疾病的发病机理,免疫逃逸和药物开发的理解具有潜在的意义。剖析所涉及的机制将是一项重要的实验挑战。











































 京公网安备 11010802027423号
京公网安备 11010802027423号