当前位置:
X-MOL 学术
›
Phytomedicine
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Limonin ameliorates acetaminophen-induced hepatotoxicity by activating Nrf2 antioxidative pathway and inhibiting NF-κB inflammatory response via upregulating Sirt1.
Phytomedicine ( IF 6.7 ) Pub Date : 2020-03-20 , DOI: 10.1016/j.phymed.2020.153211 Runyu Yang 1 , Changqin Song 1 , Jiaxi Chen 1 , Lvqi Zhou 1 , Xiubo Jiang 1 , Xiaomei Cao 2 , Yang Sun 3 , Qi Zhang 3
Phytomedicine ( IF 6.7 ) Pub Date : 2020-03-20 , DOI: 10.1016/j.phymed.2020.153211 Runyu Yang 1 , Changqin Song 1 , Jiaxi Chen 1 , Lvqi Zhou 1 , Xiubo Jiang 1 , Xiaomei Cao 2 , Yang Sun 3 , Qi Zhang 3
Affiliation
|
BACKGROUND
Limonin, a bioactive compound from citrus plants, exerts antioxidant activities, however its therapeutic potential in acetaminophen (APAP)-induced hepatotoxicity remains unclear.
PURPOSE
Our study aims to investigate the protective effect of limonin on APAP-induced hepatotoxicity and illuminate the underlying mechanisms.
STUDY
design In vitro, we chose L-02 cells to establish in vitro APAP-induced liver injury model. L-02 cells were treated with APAP (7.5 mM) for 24 h after pre-incubation with limonin (10, 25, 50 μM) or NAC (250 μM) for 2 h. In vivo, we used C57BL/6 mice as an in vivo APAP-induced liver injury model. C57BL/6 mice with pre-treatment of limonin (40, 80 mg/kg) or NAC (150 mg/kg) for 1 h, were given with a single dose of APAP (300 mg/kg).
METHODS
After pre-incubation with limonin (10, 25, 50 μM) for 2 h, L-02 cells were treated with APAP (7.5 mM) for 24 h.The experiments in vitro included MTT assay, Annexin V/PI staining, measurement of reactive oxygen species (ROS), quantitative real-time PCR analysis, Western blot analysis, immunofluorescence microscopy and analysis of LDH activity. Transfection of Nrf2 or Sirt1 siRNA was also conducted in vitro. In vivo, C57BL/6 mice with pre-treatment of limonin (40, 80 mg/kg) or NAC (150 mg/kg) for 1 h, were given with a single dose of APAP (300 mg/kg). Mice were sacrificed at 4, 12 h after APAP poisoning, and analysis of ALT and AST in serum, GSH level in liver tissues, liver histological observation and immunohistochemistry were performed.
RESULTS
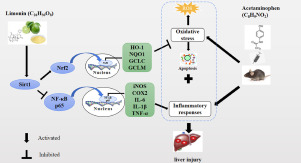
Limonin increased the cell viability and alleviated APAP-induced apoptosis in hepatocytes. Limonin also inhibited APAP-induced mitochondrial-mediated apoptosis by decreasing the ratio of Bax/Bcl-2, recovery of mitochondrial membrane potential (MMP), inhibiting ROS production and cleavage of caspase-3 in L-02 cells. Moreover, limonin induced activation of Nrf2 and increased protein expression and mRNA levels of its downstream targets, including HO-1, NQO1 and GCLC/GCLM. The inhibition of limonin on apoptosis and promotion on Nrf2 antioxidative pathway were lessened after the application of Nrf2 siRNA. In addition, limonin inhibited NF-κB transcriptional activation, NF-κB-regulated genes and protein expression of inflammatory related proteins iNOS and COX2. Furthermore, limonin increased the protein expression of Sirt1. Sirt1 siRNA transfection confirmed that limonin activated Nrf2 antioxidative pathway and inhibited NF-κB inflammatory response by upregulating Sirt1. Finally, we established APAP-induced liver injury in vivo and demonstrated that limonin alleviated APAP-induced hepatotoxicity by activating Nrf2 antioxidative signals and inhibiting NF-κB inflammatory response via upregulating Sirt1.
CONCLUSION
In summary, this study documented that limonin mitigated APAP-induced hepatotoxicity by activating Nrf2 antioxidative pathway and inhibiting NF-κB inflammatory response via upregulating Sirt1, and demonstrated that limonin had therapeutic promise in APAP-induced liver injury.
中文翻译:

柠檬苦素通过激活Nrf2抗氧化途径并通过上调Sirt1抑制NF-κB炎症反应来改善对乙酰氨基酚引起的肝毒性。
背景技术柠檬苦素是一种来自柑橘类植物的生物活性化合物,具有抗氧化作用,但是其对乙酰氨基酚(APAP)诱导的肝毒性的治疗潜力仍不清楚。目的我们的研究旨在研究柠檬苦素对APAP诱导的肝毒性的保护作用,并阐明其潜在机制。研究设计在体外,我们选择L-02细胞建立体外APAP诱导的肝损伤模型。在与柠檬苦素(10、25、50μM)或NAC(250μM)预温育2 h后,将L-02细胞用APAP(7.5 mM)处理24 h。在体内,我们将C57BL / 6小鼠用作体内APAP诱导的肝损伤模型。给予柠檬苦素(40,80 mg / kg)或NAC(150 mg / kg)预处理1小时的C57BL / 6小鼠,给予单剂量的APAP(300 mg / kg)。方法与柠檬苦素(10、25、50μM)预孵育2小时后,L-02细胞用APAP(7.5 mM)处理24 h。体外实验包括MTT分析,膜联蛋白V / PI染色,活性氧(ROS)测定,实时荧光定量PCR分析,蛋白质印迹分析,免疫荧光显微镜检查和LDH活性分析。Nrf2或Sirt1 siRNA的转染也在体外进行。在体内,用柠檬素(40,80 mg / kg)或NAC(150 mg / kg)预处理1小时的C57BL / 6小鼠接受单剂量的APAP(300 mg / kg)。在APAP中毒后第4、12 h处死小鼠,并分析血清ALT和AST,肝组织GSH水平,肝组织学观察和免疫组化。结果柠檬苦素增加了细胞活力并减轻了APAP诱导的肝细胞凋亡。柠檬苦素还通过降低Bax / Bcl-2的比率,线粒体膜电位(MMP)的恢复,抑制ROS的产生以及L-02细胞中caspase-3的裂解来抑制APAP诱导的线粒体介导的凋亡。此外,柠檬苦素诱导Nrf2活化并增加其下游靶标(包括HO-1,NQO1和GCLC / GCLM)的蛋白质表达和mRNA水平。应用Nrf2 siRNA后,柠檬苦素对细胞凋亡的抑制作用和Nrf2抗氧化途径的促进作用减弱。此外,柠檬苦素还抑制炎症相关蛋白iNOS和COX2的NF-κB转录激活,NF-κB调控的基因和蛋白表达。此外,柠檬苦素增加Sirt1的蛋白质表达。Sirt1 siRNA转染证实柠檬苦素通过上调Sirt1激活Nrf2抗氧化途径并抑制NF-κB炎症反应。最后,我们建立了APAP诱导的体内肝损伤,并证明柠檬苦素通过激活Nrf2抗氧化信号并通过上调Sirt1抑制NF-κB炎症反应来减轻APAP诱导的肝毒性。结论总的来说,该研究证明柠檬苦素通过上调Sirt1激活Nrf2抗氧化途径并抑制NF-κB炎症反应,从而减轻APAP诱导的肝毒性,并证明柠檬苦素对APAP诱导的肝损伤具有治疗前景。我们建立了APAP诱导的体内肝损伤,并证明柠檬苦素通过激活Nrf2抗氧化信号并通过上调Sirt1抑制NF-κB炎症反应来减轻APAP诱导的肝毒性。结论总的来说,该研究证明柠檬苦素通过上调Sirt1激活Nrf2抗氧化途径并抑制NF-κB炎症反应,从而减轻APAP诱导的肝毒性,并证明柠檬苦素对APAP诱导的肝损伤具有治疗前景。我们建立了APAP诱导的体内肝损伤,并证明柠檬苦素通过激活Nrf2抗氧化信号并通过上调Sirt1抑制NF-κB炎症反应来减轻APAP诱导的肝毒性。结论总的来说,该研究表明柠檬苦素通过激活Nrf2抗氧化途径并通过上调Sirt1抑制NF-κB炎症反应来减轻APAP诱导的肝毒性,并证明柠檬苦素对APAP诱导的肝损伤具有治疗前景。
更新日期:2020-03-21
中文翻译:

柠檬苦素通过激活Nrf2抗氧化途径并通过上调Sirt1抑制NF-κB炎症反应来改善对乙酰氨基酚引起的肝毒性。
背景技术柠檬苦素是一种来自柑橘类植物的生物活性化合物,具有抗氧化作用,但是其对乙酰氨基酚(APAP)诱导的肝毒性的治疗潜力仍不清楚。目的我们的研究旨在研究柠檬苦素对APAP诱导的肝毒性的保护作用,并阐明其潜在机制。研究设计在体外,我们选择L-02细胞建立体外APAP诱导的肝损伤模型。在与柠檬苦素(10、25、50μM)或NAC(250μM)预温育2 h后,将L-02细胞用APAP(7.5 mM)处理24 h。在体内,我们将C57BL / 6小鼠用作体内APAP诱导的肝损伤模型。给予柠檬苦素(40,80 mg / kg)或NAC(150 mg / kg)预处理1小时的C57BL / 6小鼠,给予单剂量的APAP(300 mg / kg)。方法与柠檬苦素(10、25、50μM)预孵育2小时后,L-02细胞用APAP(7.5 mM)处理24 h。体外实验包括MTT分析,膜联蛋白V / PI染色,活性氧(ROS)测定,实时荧光定量PCR分析,蛋白质印迹分析,免疫荧光显微镜检查和LDH活性分析。Nrf2或Sirt1 siRNA的转染也在体外进行。在体内,用柠檬素(40,80 mg / kg)或NAC(150 mg / kg)预处理1小时的C57BL / 6小鼠接受单剂量的APAP(300 mg / kg)。在APAP中毒后第4、12 h处死小鼠,并分析血清ALT和AST,肝组织GSH水平,肝组织学观察和免疫组化。结果柠檬苦素增加了细胞活力并减轻了APAP诱导的肝细胞凋亡。柠檬苦素还通过降低Bax / Bcl-2的比率,线粒体膜电位(MMP)的恢复,抑制ROS的产生以及L-02细胞中caspase-3的裂解来抑制APAP诱导的线粒体介导的凋亡。此外,柠檬苦素诱导Nrf2活化并增加其下游靶标(包括HO-1,NQO1和GCLC / GCLM)的蛋白质表达和mRNA水平。应用Nrf2 siRNA后,柠檬苦素对细胞凋亡的抑制作用和Nrf2抗氧化途径的促进作用减弱。此外,柠檬苦素还抑制炎症相关蛋白iNOS和COX2的NF-κB转录激活,NF-κB调控的基因和蛋白表达。此外,柠檬苦素增加Sirt1的蛋白质表达。Sirt1 siRNA转染证实柠檬苦素通过上调Sirt1激活Nrf2抗氧化途径并抑制NF-κB炎症反应。最后,我们建立了APAP诱导的体内肝损伤,并证明柠檬苦素通过激活Nrf2抗氧化信号并通过上调Sirt1抑制NF-κB炎症反应来减轻APAP诱导的肝毒性。结论总的来说,该研究证明柠檬苦素通过上调Sirt1激活Nrf2抗氧化途径并抑制NF-κB炎症反应,从而减轻APAP诱导的肝毒性,并证明柠檬苦素对APAP诱导的肝损伤具有治疗前景。我们建立了APAP诱导的体内肝损伤,并证明柠檬苦素通过激活Nrf2抗氧化信号并通过上调Sirt1抑制NF-κB炎症反应来减轻APAP诱导的肝毒性。结论总的来说,该研究证明柠檬苦素通过上调Sirt1激活Nrf2抗氧化途径并抑制NF-κB炎症反应,从而减轻APAP诱导的肝毒性,并证明柠檬苦素对APAP诱导的肝损伤具有治疗前景。我们建立了APAP诱导的体内肝损伤,并证明柠檬苦素通过激活Nrf2抗氧化信号并通过上调Sirt1抑制NF-κB炎症反应来减轻APAP诱导的肝毒性。结论总的来说,该研究表明柠檬苦素通过激活Nrf2抗氧化途径并通过上调Sirt1抑制NF-κB炎症反应来减轻APAP诱导的肝毒性,并证明柠檬苦素对APAP诱导的肝损伤具有治疗前景。











































 京公网安备 11010802027423号
京公网安备 11010802027423号