当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An in vitro and in vivo study of the brain-targeting effects of an epidermal growth factor-functionalized cholera toxin-like chimeric protein.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-03-20 , DOI: 10.1016/j.jconrel.2020.03.027 Huafeng He 1 , Danmin Lin 1 , Jiajie Sun 1 , Xianying He 1 , Tiantian Wang 1 , Yinglin Fang 1 , Yijun Liu 1 , Kaixiang Fan 1 , Xinxin Chen 1 , Huahong He 2 , Xiangguang Li 1 , Biansheng Ji 3 , Suqing Zhao 1 , Xi Zheng 1 , Kun Zhang 4 , Huaqian Wang 1
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-03-20 , DOI: 10.1016/j.jconrel.2020.03.027 Huafeng He 1 , Danmin Lin 1 , Jiajie Sun 1 , Xianying He 1 , Tiantian Wang 1 , Yinglin Fang 1 , Yijun Liu 1 , Kaixiang Fan 1 , Xinxin Chen 1 , Huahong He 2 , Xiangguang Li 1 , Biansheng Ji 3 , Suqing Zhao 1 , Xi Zheng 1 , Kun Zhang 4 , Huaqian Wang 1
Affiliation
|
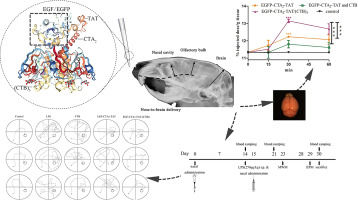
The development of neuroprotective drugs has proven to be extremely difficult because of the blood-brain barrier. Intranasal administration is thought to transport the drug from the nasal cavity along the olfactory and trigeminal nerves to the brain, thus bypassing the blood-brain barrier. However, macromolecular protein drugs have low delivery efficiency via this route in general. We hypothesized that an innocuous cholera toxin-like chimeric protein could better enhance the efficiency of protein delivery through the intranasal route. To test this hypothesis, we designed an enhanced green fluorescent protein (EGFP) chimera to evaluate the effect of the cholera toxin (CT) as a carrier for drug delivery into the brain. Then, the EGFP was replaced with epidermal growth factor (EGF) in the chimeric protein, and the therapeutic effect of the new chimeric protein was studied in an LPS-induced neuritis mouse model. The results suggest that the CT-like chimeric protein can bypass the blood-brain barrier and enter the brain in approximately 30 min. This EGF chimeric protein can effectively protect the spatial cognitive ability of and confer anti-anxiety protection to mice. The results indicate that cholera toxin-like chimeric proteins are potential tools for effectively delivering macromodecular drugs into the brain through intranasal administration.
中文翻译:

对表皮生长因子功能化的霍乱毒素样嵌合蛋白的脑靶向作用的体外和体内研究。
由于血脑屏障,已证明神经保护药物的开发非常困难。鼻内给药被认为是将药物从鼻腔沿着嗅觉和三叉神经传递到大脑,从而绕过了血脑屏障。然而,大分子蛋白药物通常通过这种途径具有低的递送效率。我们假设无毒的霍乱毒素样嵌合蛋白可以更好地增强通过鼻内途径递送蛋白的效率。为了验证该假设,我们设计了增强的绿色荧光蛋白(EGFP)嵌合体,以评估霍乱毒素(CT)作为药物向大脑输送的载体的作用。然后,将嵌合蛋白中的表皮生长因子(EGF)替换为EGFP,在LPS诱导的神经炎小鼠模型中研究了新的嵌合蛋白的治疗效果。结果表明,类似CT的嵌合蛋白可以绕过血脑屏障并在大约30分钟内进入大脑。这种EGF嵌合蛋白可以有效地保护小鼠的空间认知能力并赋予其抗焦虑保护作用。结果表明,霍乱毒素样嵌合蛋白是通过鼻内给药有效地将大分子药物输送到大脑的潜在工具。这种EGF嵌合蛋白可以有效地保护小鼠的空间认知能力并赋予其抗焦虑保护作用。结果表明,霍乱毒素样嵌合蛋白是通过鼻内给药有效地将大分子药物输送到大脑的潜在工具。这种EGF嵌合蛋白可以有效地保护小鼠的空间认知能力并赋予其抗焦虑保护作用。结果表明,霍乱毒素样嵌合蛋白是通过鼻内给药有效地将大分子药物输送到大脑的潜在工具。
更新日期:2020-03-21
中文翻译:

对表皮生长因子功能化的霍乱毒素样嵌合蛋白的脑靶向作用的体外和体内研究。
由于血脑屏障,已证明神经保护药物的开发非常困难。鼻内给药被认为是将药物从鼻腔沿着嗅觉和三叉神经传递到大脑,从而绕过了血脑屏障。然而,大分子蛋白药物通常通过这种途径具有低的递送效率。我们假设无毒的霍乱毒素样嵌合蛋白可以更好地增强通过鼻内途径递送蛋白的效率。为了验证该假设,我们设计了增强的绿色荧光蛋白(EGFP)嵌合体,以评估霍乱毒素(CT)作为药物向大脑输送的载体的作用。然后,将嵌合蛋白中的表皮生长因子(EGF)替换为EGFP,在LPS诱导的神经炎小鼠模型中研究了新的嵌合蛋白的治疗效果。结果表明,类似CT的嵌合蛋白可以绕过血脑屏障并在大约30分钟内进入大脑。这种EGF嵌合蛋白可以有效地保护小鼠的空间认知能力并赋予其抗焦虑保护作用。结果表明,霍乱毒素样嵌合蛋白是通过鼻内给药有效地将大分子药物输送到大脑的潜在工具。这种EGF嵌合蛋白可以有效地保护小鼠的空间认知能力并赋予其抗焦虑保护作用。结果表明,霍乱毒素样嵌合蛋白是通过鼻内给药有效地将大分子药物输送到大脑的潜在工具。这种EGF嵌合蛋白可以有效地保护小鼠的空间认知能力并赋予其抗焦虑保护作用。结果表明,霍乱毒素样嵌合蛋白是通过鼻内给药有效地将大分子药物输送到大脑的潜在工具。









































 京公网安备 11010802027423号
京公网安备 11010802027423号