Environment International ( IF 10.3 ) Pub Date : 2020-03-20 , DOI: 10.1016/j.envint.2020.105659 Wei Yan , Huifeng Yue , Xiaotong Ji , Guangke Li , Nan Sang
|
Background
Early-life exposure to nitrogen dioxide (NO2) is associated with an increased risk of developing a neurodevelopmental disorder during childhood or later in life.
Objectives
We investigated whether prenatal NO2 inhalation causes neurodevelopmental abnormalities and cognitive deficits in weanling offspring without subsequent postnatal NO2 exposure and how this prenatal exposure contributes to postnatal consequences.
Methods
Pregnant C57BL/6 mice were exposed to air or NO2 (2.5 ppm, 5 h/day) throughout gestation, and the offspring were sacrificed on postnatal days (PNDs) 1, 7, 14 and 21. We determined the mRNA profiles of different postnatal developmental windows, detected the long noncoding RNA (lncRNA) profiles and cognitive function in weanling offspring, and analyzed the effects of hub lncRNAs on differentially expressed genes (DEGs).
Results
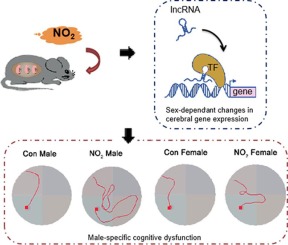
Prenatal NO2 inhalation significantly impaired cognitive function in the weanling male, but not female, offspring. The male-specific response was coupled with abnormal neuropathologies and transcriptional profiles in the cortex during different postnatal developmental windows. Consistently, Gene Ontology (GO) analysis of the DEGs revealed persistent disruptions in neurodevelopment-associated biological processes and cellular components in the male offspring, and Apolipoprotein E (ApoE) was one of key factors contributing to prenatal exposure-induced male-specific neurological dysfunction. In addition, distinct sex-dependent lncRNA expression was identified in the weanling offspring, and metastasis-associated lung adenocarcinoma transcript 1 (Malat1) acted as a hub lncRNA and was coexpressed with most coding genes in the lncRNA-mRNA coexpressed pairs in the male offspring. Importantly, lncRNA Malat1 expression was elevated, and Malat1 modulated ApoE expression through NF-κB activation during this process.
Conclusions
Prenatal NO2 exposure is related to sex-dependent neurocognitive deficits and transcriptomic profile changes in the cortices of the prenatally exposed offspring. Male-specific neurological dysfunction is associated with the constant alteration of genes during postnatal neurodevelopment and their transcriptional modulation by hub lncRNAs.
中文翻译:

子代小鼠的产前NO 2暴露和神经发育障碍:转录组学揭示了大脑基因表达的性别依赖性变化
背景
生命早期暴露于二氧化氮(NO 2)与儿童时期或人生后期发展成神经发育障碍的风险增加有关。
目标
我们调查了在没有随后的出生后NO 2暴露的情况下,吸入产前NO 2吸入是否会引起断奶后代的神经发育异常和认知缺陷,以及这种产前暴露如何导致出生后后果。
方法
在整个妊娠期间,将怀孕的C57BL / 6小鼠暴露于空气或NO 2(2.5 ppm,5 h / day)中,并在出生后的第1、7、14和21天处死后代。我们确定了不同基因的mRNA谱图产后发育窗口,检测了断奶后代的长非编码RNA(lncRNA)概况和认知功能,并分析了轮毂lncRNA对差异表达基因(DEG)的影响。
结果
产前NO 2吸入会严重损害断奶的雄性而非雌性后代的认知功能。在不同的产后发育期中,男性特异性反应与异常的神经病理学和皮层中的转录特征相结合。一致地,对DEG的基因本体论(GO)分析显示,雄性后代中与神经发育相关的生物过程和细胞成分持续受到破坏,载脂蛋白E(ApoE)是导致产前暴露诱发的雄性特定神经功能障碍的关键因素之一。 。此外,在断奶的后代中发现了独特的性别依赖性lncRNA表达,与转移相关的肺腺癌转录本1(Malat1)充当枢纽lncRNA,并在雄性后代中与lncRNA-mRNA共表达对中的大多数编码基因共表达。重要的是,在此过程中,lncRNA Malat1表达升高,而Malat1通过NF-κB激活调节ApoE表达。
结论
产前NO 2暴露与产前暴露后代皮层中性别相关的神经认知缺陷和转录组谱变化有关。男性特有的神经功能障碍与产后神经发育过程中基因的不断变化及其中枢lncRNA的转录调控有关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号