当前位置:
X-MOL 学术
›
Eur. J. Pharm. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
High payload and targeted release of anthracyclines by aptamer-tethered DNA nanotrains - Thermodynamic and release kinetic study.
European Journal of Pharmaceutical Sciences ( IF 4.6 ) Pub Date : 2020-03-20 , DOI: 10.1016/j.ejps.2020.105319 Wenxin Pei 1 , Min Liu 2 , Yushu Wu 1 , Yanna Zhao 1 , Tingting Liu 1 , Bin Sun 1 , Yinglin Liu 3 , Qingpeng Wang 1 , Jun Han 1
European Journal of Pharmaceutical Sciences ( IF 4.6 ) Pub Date : 2020-03-20 , DOI: 10.1016/j.ejps.2020.105319 Wenxin Pei 1 , Min Liu 2 , Yushu Wu 1 , Yanna Zhao 1 , Tingting Liu 1 , Bin Sun 1 , Yinglin Liu 3 , Qingpeng Wang 1 , Jun Han 1
Affiliation
|
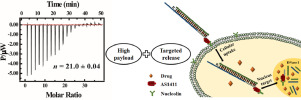
As one of the most promising drug delivery carriers, self-assembled DNA nanostructures are characterized of well-defined sizes, excellent biocompatibility, high drug loading and ability to control drug release. Studying the interactions between anticancer drugs and DNA nanostructures can help to associate microstructure-drug loading-release rate-therapeutic effect. Herein AS1411 aptamer-tethered DNA nanotrains (AS1411NTrs) were constructed and used as anthracyclines carrier with high payload for targeted delivery. The bindings of doxorubicin (DOX), epirubicin (EPI), and daunorubicin (DAU) to AS1411NTrs were investigated by isothermal titration calorimetry and fluorescence spectroscopy, and thermodynamic parameters were obtained. The high drug payload capacity of AS1411NTrs was verified by the large number of binding sites (~20). The binding mode was determined by differential scanning calorimetry and potassium iodide (KI) quenching experiments. The release experiment data showed that DNase I facilitated drug release and the release followed the first-order kinetic model. MTT cell viability assay demonstrated that the drug-loaded AS1411NTrs had significantly higher cytotoxicity against target HeLa cells than normal human liver L02 cells. These findings revealed that AS1411NTrs had high payload and targeted release capacity for DOX, EPI, and DAU. This result can provide a theoretical basis for constructing reasonable DNA nanostructures based on drug carriers.
中文翻译:

适体束缚的DNA纳米火车的高有效载荷和蒽环类药物的靶向释放-热力学和释放动力学研究。
自组装DNA纳米结构作为最有希望的药物传递载体之一,具有确定的尺寸,出色的生物相容性,高载药量和控制药物释放的能力。研究抗癌药物和DNA纳米结构之间的相互作用可以帮助关联微结构-药物加载-释放速率-治疗效果。本文中,构建了AS1411适体拴系的DNA纳米序列(AS1411NTrs),并用作具有高负载量的蒽环类载体,用于靶向递送。通过等温滴定热分析和荧光光谱法研究了阿霉素(DOX),表柔比星(EPI)和柔红霉素(DAU)与AS1411NTrs的结合,并获得了热力学参数。大量的结合位点(〜20个)证实了AS1411NTrs的高药物有效负载能力。通过差示扫描量热法和碘化钾(KI)淬灭实验确定结合模式。释放实验数据表明,DNase I促进了药物释放,并且释放遵循一级动力学模型。MTT细胞生存力分析表明,载药的AS1411NTrs与正常人肝L02细胞相比,对目标HeLa细胞具有明显更高的细胞毒性。这些发现表明,AS1411NTrs对于DOX,EPI和DAU具有较高的有效负载和目标释放能力。该结果可为基于药物载体构建合理的DNA纳米结构提供理论依据。释放实验数据表明,DNase I促进了药物释放,并且释放遵循一级动力学模型。MTT细胞生存力分析表明,载药的AS1411NTrs与正常人肝L02细胞相比,对目标HeLa细胞具有明显更高的细胞毒性。这些发现表明,AS1411NTrs对于DOX,EPI和DAU具有较高的有效负载和目标释放能力。该结果可为基于药物载体构建合理的DNA纳米结构提供理论依据。释放实验数据表明,DNase I促进了药物释放,并且释放遵循一级动力学模型。MTT细胞生存力分析表明,载药的AS1411NTrs与正常人肝L02细胞相比,对目标HeLa细胞具有明显更高的细胞毒性。这些发现表明,AS1411NTrs对于DOX,EPI和DAU具有较高的有效负载和目标释放能力。该结果可为基于药物载体构建合理的DNA纳米结构提供理论依据。
更新日期:2020-03-21
中文翻译:

适体束缚的DNA纳米火车的高有效载荷和蒽环类药物的靶向释放-热力学和释放动力学研究。
自组装DNA纳米结构作为最有希望的药物传递载体之一,具有确定的尺寸,出色的生物相容性,高载药量和控制药物释放的能力。研究抗癌药物和DNA纳米结构之间的相互作用可以帮助关联微结构-药物加载-释放速率-治疗效果。本文中,构建了AS1411适体拴系的DNA纳米序列(AS1411NTrs),并用作具有高负载量的蒽环类载体,用于靶向递送。通过等温滴定热分析和荧光光谱法研究了阿霉素(DOX),表柔比星(EPI)和柔红霉素(DAU)与AS1411NTrs的结合,并获得了热力学参数。大量的结合位点(〜20个)证实了AS1411NTrs的高药物有效负载能力。通过差示扫描量热法和碘化钾(KI)淬灭实验确定结合模式。释放实验数据表明,DNase I促进了药物释放,并且释放遵循一级动力学模型。MTT细胞生存力分析表明,载药的AS1411NTrs与正常人肝L02细胞相比,对目标HeLa细胞具有明显更高的细胞毒性。这些发现表明,AS1411NTrs对于DOX,EPI和DAU具有较高的有效负载和目标释放能力。该结果可为基于药物载体构建合理的DNA纳米结构提供理论依据。释放实验数据表明,DNase I促进了药物释放,并且释放遵循一级动力学模型。MTT细胞生存力分析表明,载药的AS1411NTrs与正常人肝L02细胞相比,对目标HeLa细胞具有明显更高的细胞毒性。这些发现表明,AS1411NTrs对于DOX,EPI和DAU具有较高的有效负载和目标释放能力。该结果可为基于药物载体构建合理的DNA纳米结构提供理论依据。释放实验数据表明,DNase I促进了药物释放,并且释放遵循一级动力学模型。MTT细胞生存力分析表明,载药的AS1411NTrs与正常人肝L02细胞相比,对目标HeLa细胞具有明显更高的细胞毒性。这些发现表明,AS1411NTrs对于DOX,EPI和DAU具有较高的有效负载和目标释放能力。该结果可为基于药物载体构建合理的DNA纳米结构提供理论依据。



























 京公网安备 11010802027423号
京公网安备 11010802027423号