Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2020-03-20 , DOI: 10.1016/j.apcata.2020.117512 Zhihui Wang , Bingbing Chen , Mark Crocker , Limei Yu , Chuan Shi
|
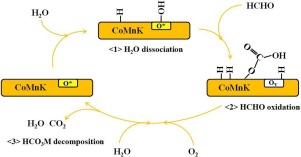
Although CoMn oxides have shown promising catalytic properties in HCHO oxidation, their activities have a large space to improve especially at low temperatures (<70 °C). In the present study, Na or K doping into CoMn oxides was found to greatly enhance the low temperature activities, being one of the most active catalysts among non-noble metal catalysts. Na or K doping into CoMn oxides generates additional surface adsorbed oxygen and the amount of which has a linear relationship with the HCHO oxidation rate at 30 °C and 40 °C, which gives clear evidence of the crucial roles of surface adsorbed oxygen in HCHO oxidation. What is more, the positive effect of K doping was boosted when H2O was co-existed in the feed gas. The surface hydroxyl species generated from H2O dissociation directly participate in the HCHO oxidation, which provides a low activation energy pathway and increase the low temperature activity.
中文翻译:

碱金属改性的CoMn-氧化物催化剂在低温下甲醛氧化的新见解
尽管CoMn氧化物在HCHO氧化中显示出令人鼓舞的催化性能,但它们的活性仍有很大的提升空间,尤其是在低温(<70°C)下。在本研究中,发现向CoMn氧化物中掺入Na或K可以极大地增强低温活性,这是非贵金属催化剂中活性最高的催化剂之一。将Na或K掺杂到CoMn氧化物中会产生额外的表面吸附氧,其含量与30°C和40°C下HCHO的氧化速率呈线性关系,这清楚地证明了表面吸附的氧在HCHO氧化中的关键作用。此外,当原料气中共存有H 2 O时,K掺杂的积极作用得到增强。H 2产生的表面羟基物质O解离直接参与HCHO氧化,这提供了低活化能途径并增加了低温活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号