当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Some Physicochemical Properties of Binary Mixtures of Glycerol with Butanol Isomers in the Temperature Range 293.15–318.15 K and Ambient Pressure
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-03-20 , DOI: 10.1021/acs.jced.9b01052 Yasmine Chabouni 1 , Fouzia Amireche 1, 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-03-20 , DOI: 10.1021/acs.jced.9b01052 Yasmine Chabouni 1 , Fouzia Amireche 1, 2
Affiliation
|
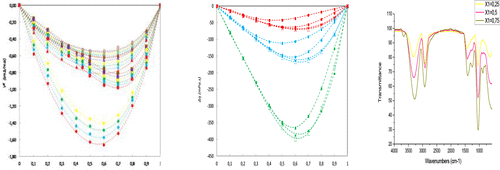
The aim of this work is experimental determination of density, ρ, refractive index, nD, viscosity, η, and their derivatives for four binary systems containing glycerol in the mixture with butanol isomers: 1-butanol, 2-butanol, isobutanol, and tert-butanol. Experimental results were carried out for all concentration ranges at atmospheric pressure and at a temperature interval of 293.15–318.15 K except for the mixtures with tert-butanol because of high melting temperatures, so the measurements were made from 303 K. Results of experimental measurements were further used to estimate, for each investigated mixture, excess molar volumes, partial molar volumes, apparent molar volumes, thermal isobaric expansibilities, excess thermal expansibilities and partial molar volumes at infinite dilution, the viscosity deviations, and the excess energies of activation. In addition to that, the infrared spectra of all the systems have been recorded at three concentrations at room temperature. The excess molar volumes VE and excess thermal expansitivities for all investigated systems are found negative over the whole composition range and at all temperatures studied, and deviations from ideality increase with increasing temperature. Negative values of viscosity deviation appear for all systems, but contrary to the excess molar volumes, the deviations from ideality decrease with temperature rising. The excess molar calculated properties were well fitted using the empirical Redlich–Kister polynomial. All the properties measured and calculated in this work have shown a significant influence of the structure of the molecules including the size, shape, and the position of the hydroxyl groups of the components. As expected, the infrared spectra of these binary mixtures prepared with these four butanol isomers show strong hydrogen bonding capability.
中文翻译:

293.15–318.15 K和环境压力下甘油与丁醇异构体的二元混合物的一些理化性质
这个工作的目的是密度,ρ,折射率,的实验测定Ñ d,粘度,η,以及它们的衍生物为含有与丁醇异构体的混合物的甘油四个二进制系统:1-丁醇,2-丁醇,异丁醇,和叔丁醇。在大气压和293.15–318.15 K的温度范围内,对所有浓度范围进行了实验,但与叔酸的混合物除外-丁醇由于熔化温度高,所以测量是从303 K开始的。实验测量结果还用于估算每种混合物的过量摩尔体积,部分摩尔体积,表观摩尔体积,热等压膨胀性,过量热无限稀释时的膨胀性和部分摩尔体积,粘度偏差以及活化的多余能量。除此之外,在室温下以三种浓度记录了所有系统的红外光谱。多余的摩尔体积V E在整个研究范围内和所有温度下,所有被研究系统的过量热膨胀系数均为负,并且随着温度的升高,与理想度的偏差也会增加。对于所有系统,粘度偏差均为负值,但与过量的摩尔体积相反,与理想值的偏差随温度升高而减小。使用经验Redlich-Kister多项式可以很好地拟合出多余的摩尔计算的特性。在这项工作中测量和计算的所有特性均显示出对分子结构的重大影响,包括组分的大小,形状和羟基位置。如预期的那样,用这四种丁醇异构体制备的这些二元混合物的红外光谱显示出很强的氢键能力。
更新日期:2020-04-24
中文翻译:

293.15–318.15 K和环境压力下甘油与丁醇异构体的二元混合物的一些理化性质
这个工作的目的是密度,ρ,折射率,的实验测定Ñ d,粘度,η,以及它们的衍生物为含有与丁醇异构体的混合物的甘油四个二进制系统:1-丁醇,2-丁醇,异丁醇,和叔丁醇。在大气压和293.15–318.15 K的温度范围内,对所有浓度范围进行了实验,但与叔酸的混合物除外-丁醇由于熔化温度高,所以测量是从303 K开始的。实验测量结果还用于估算每种混合物的过量摩尔体积,部分摩尔体积,表观摩尔体积,热等压膨胀性,过量热无限稀释时的膨胀性和部分摩尔体积,粘度偏差以及活化的多余能量。除此之外,在室温下以三种浓度记录了所有系统的红外光谱。多余的摩尔体积V E在整个研究范围内和所有温度下,所有被研究系统的过量热膨胀系数均为负,并且随着温度的升高,与理想度的偏差也会增加。对于所有系统,粘度偏差均为负值,但与过量的摩尔体积相反,与理想值的偏差随温度升高而减小。使用经验Redlich-Kister多项式可以很好地拟合出多余的摩尔计算的特性。在这项工作中测量和计算的所有特性均显示出对分子结构的重大影响,包括组分的大小,形状和羟基位置。如预期的那样,用这四种丁醇异构体制备的这些二元混合物的红外光谱显示出很强的氢键能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号