当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Designing an Artificial Pathway for the Biosynthesis of a Novel Phenazine N-Oxide in Pseudomonas chlororaphis HT66.
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-03-26 , DOI: 10.1021/acssynbio.9b00515 Shuqi Guo 1 , Rongfeng Liu 1 , Wei Wang 1 , Hongbo Hu 1, 2 , Zhiyong Li 1 , Xuehong Zhang 1
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-03-26 , DOI: 10.1021/acssynbio.9b00515 Shuqi Guo 1 , Rongfeng Liu 1 , Wei Wang 1 , Hongbo Hu 1, 2 , Zhiyong Li 1 , Xuehong Zhang 1
Affiliation
|
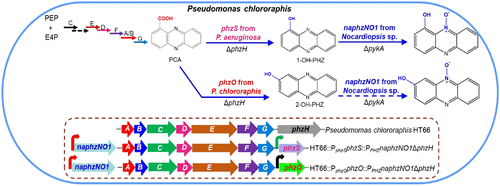
Aromatic N-oxides are valuable due to their versatile chemical, pharmaceutical, and agricultural applications. Natural phenazine N-oxides possess potent biological activities and can be applied in many ways; however, few N-oxides have been identified. Herein, we developed a microbial system to synthesize phenazine N-oxides via an artificial pathway. First, the N-monooxygenase NaphzNO1 was predicted and screened in Nocardiopsis sp. 13-12-13 through a product comparison and gene sequencing. Subsequently, according to similarities in the chemical structures of substrates, an artificial pathway for the synthesis of a phenazine N-oxide in Pseudomonas chlororaphis HT66 was designed and established using three heterologous enzymes, a monooxygenase (PhzS) from P. aeruginosa PAO1, a monooxygenase (PhzO) from P. chlororaphis GP72, and the N-monooxygenase NaphzNO1. A novel phenazine derivative, 1-hydroxyphenazine N'10-oxide, was obtained in an engineered strain, P. chlororaphis HT66-SN. The phenazine N-monooxygenase NaphzNO1 was identified by metabolically engineering the phenazine-producing platform P. chlororaphis HT66. Moreover, the function of NaphzNO1, which can catalyze the conversion of 1-hydroxyphenazine but not that of 2-hydroxyphenazine, was confirmed in vitro. Additionally, 1-hydroxyphenazine N'10-oxide demonstrated substantial cytotoxic activity against two human cancer cell lines, MCF-7 and HT-29. Furthermore, the highest microbial production of 1-hydroxyphenazine N'10-oxide to date was achieved at 143.4 mg/L in the metabolically engineered strain P3-SN. These findings demonstrate that P. chlororaphis HT66 has the potential to be engineered as a platform for phenazine-modifying gene identification and derivative production. The present study also provides a promising alternative for the sustainable synthesis of aromatic N-oxides with unique chemical structures by N-monooxygenase.
中文翻译:

设计一种人工途径,用于在绿假单胞菌HT66中生物合成新型吩嗪N-氧化物。
芳香族N-氧化物因其在化学,制药和农业领域的广泛用途而非常有价值。天然吩嗪N-氧化物具有强大的生物活性,可以多种方式使用;但是,几乎没有发现N氧化物。在这里,我们开发了一种通过人工途径合成吩嗪N-氧化物的微生物系统。首先,预测和筛选了N-单加氧酶NaphzNO1。13-12-13通过产品比较和基因测序。随后,根据底物化学结构的相似性,使用三种异源酶,铜绿假单胞菌PAO1的单加氧酶(PhzS),一种单加氧酶,设计并建立了在绿脓杆菌HT66中合成吩嗪N-氧化物的人工途径。 (PhzO)来自P. chlororaphis GP72,和N-单加氧酶NaphzNO1。在工程菌株P. chlororaphis HT66-SN中获得了一种新的吩嗪衍生物1-羟基吩嗪N'10-氧化物。吩嗪N-单加氧酶NaphzNO1是通过代谢改造吩嗪生产平台P. chlororaphis HT66来鉴定的。此外,在体外证实了NaphzNO1的功能可以催化1-羟基吩嗪的转化而不是2-羟基吩嗪的转化。另外,1-羟基吩嗪N'10-氧化物显示出对两种人类癌细胞系MCF-7和HT-29的实质性细胞毒活性。此外,在代谢工程菌株P3-SN中,迄今最高的1-羟基吩嗪N'10-氧化物的微生物产量为143.4 mg / L。这些发现表明P。chlorophiphis HT66有潜力被设计成可用于吩嗪修饰的基因鉴定和衍生物生产的平台。本研究还为N-单加氧酶可持续合成具有独特化学结构的芳族N-氧化物提供了有希望的替代方法。
更新日期:2020-04-23
中文翻译:

设计一种人工途径,用于在绿假单胞菌HT66中生物合成新型吩嗪N-氧化物。
芳香族N-氧化物因其在化学,制药和农业领域的广泛用途而非常有价值。天然吩嗪N-氧化物具有强大的生物活性,可以多种方式使用;但是,几乎没有发现N氧化物。在这里,我们开发了一种通过人工途径合成吩嗪N-氧化物的微生物系统。首先,预测和筛选了N-单加氧酶NaphzNO1。13-12-13通过产品比较和基因测序。随后,根据底物化学结构的相似性,使用三种异源酶,铜绿假单胞菌PAO1的单加氧酶(PhzS),一种单加氧酶,设计并建立了在绿脓杆菌HT66中合成吩嗪N-氧化物的人工途径。 (PhzO)来自P. chlororaphis GP72,和N-单加氧酶NaphzNO1。在工程菌株P. chlororaphis HT66-SN中获得了一种新的吩嗪衍生物1-羟基吩嗪N'10-氧化物。吩嗪N-单加氧酶NaphzNO1是通过代谢改造吩嗪生产平台P. chlororaphis HT66来鉴定的。此外,在体外证实了NaphzNO1的功能可以催化1-羟基吩嗪的转化而不是2-羟基吩嗪的转化。另外,1-羟基吩嗪N'10-氧化物显示出对两种人类癌细胞系MCF-7和HT-29的实质性细胞毒活性。此外,在代谢工程菌株P3-SN中,迄今最高的1-羟基吩嗪N'10-氧化物的微生物产量为143.4 mg / L。这些发现表明P。chlorophiphis HT66有潜力被设计成可用于吩嗪修饰的基因鉴定和衍生物生产的平台。本研究还为N-单加氧酶可持续合成具有独特化学结构的芳族N-氧化物提供了有希望的替代方法。










































 京公网安备 11010802027423号
京公网安备 11010802027423号