当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bioreductively Activatable Prodrug Conjugates of Combretastatin A-1 and Combretastatin A-4 as Anticancer Agents Targeted toward Tumor-Associated Hypoxia.
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-03-20 , DOI: 10.1021/acs.jnatprod.9b00773 Blake A Winn 1 , Laxman Devkota 1 , Bunnarack Kuch 1 , Matthew T MacDonough 1 , Tracy E Strecker 1 , Yifan Wang 1 , Zhe Shi 1 , Jeni L Gerberich 2 , Deboprosad Mondal 1 , Alejandro J Ramirez 3 , Ernest Hamel 4 , David J Chaplin 1, 5 , Peter Davis 5 , Ralph P Mason 2 , Mary Lynn Trawick 1 , Kevin G Pinney 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-03-20 , DOI: 10.1021/acs.jnatprod.9b00773 Blake A Winn 1 , Laxman Devkota 1 , Bunnarack Kuch 1 , Matthew T MacDonough 1 , Tracy E Strecker 1 , Yifan Wang 1 , Zhe Shi 1 , Jeni L Gerberich 2 , Deboprosad Mondal 1 , Alejandro J Ramirez 3 , Ernest Hamel 4 , David J Chaplin 1, 5 , Peter Davis 5 , Ralph P Mason 2 , Mary Lynn Trawick 1 , Kevin G Pinney 1
Affiliation
|
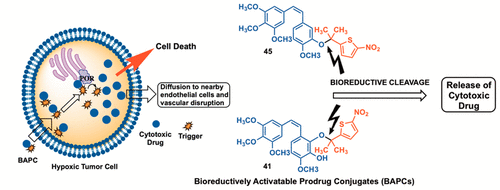
The natural products combretastatin A-1 (CA1) and combretastatin A-4 (CA4) function as potent inhibitors of tubulin polymerization and as selective vascular disrupting agents (VDAs) in tumors. Bioreductively activatable prodrug conjugates (BAPCs) can enhance selectivity by serving as substrates for reductase enzymes specifically in hypoxic regions of tumors. A series of CA1-BAPCs incorporating nor-methyl, mono-methyl, and gem-dimethyl nitrothiophene triggers were synthesized together with corresponding CA4-BAPCs, previously reported by Davis (Mol. Cancer Ther. 2006, 5 (11), 2886), for comparison. The CA4-gem-dimethylnitrothiophene BAPC 45 proved exemplary in comparison to its nor-methyl 43 and mono-methyl 44 congeners. It was stable in phosphate buffer (pH 7.4, 24 h), was cleaved (25%, 90 min) by NADPH-cytochrome P450 oxidoreductase (POR), was inactive (desirable prodrug attribute) as an inhibitor of tubulin polymerization (IC50 > 20 μM), and demonstrated hypoxia-selective activation in the A549 cell line [hypoxia cytotoxicity ratio (HCR) = 41.5]. The related CA1-gem-dimethylnitrothiophene BAPC 41 was also promising (HCR = 12.5) with complete cleavage (90 min) upon treatment with POR. In a preliminary in vivo dynamic bioluminescence imaging study, BAPC 45 (180 mg/kg, ip) induced a decrease (within 4 h) in light emission in a 4T1 syngeneic mouse breast tumor model, implying activation and vascular disruption.
中文翻译:

Combretastatin A-1 和 Combretastatin A-4 的生物还原激活前药缀合物作为针对肿瘤相关缺氧的抗癌剂。
天然产物考布他汀 A-1 (CA1) 和考布他汀 A-4 (CA4) 是微管蛋白聚合的有效抑制剂,并且是肿瘤中的选择性血管破坏剂 (VDA)。生物还原性可激活前药缀合物(BAPC)可以通过作为还原酶的底物(特别是在肿瘤缺氧区域)来增强选择性。 Davis 先前报道了一系列包含正甲基、单甲基和偕二甲基硝基噻吩触发剂的 CA1-BAPC 与相应的 CA4-BAPC 一起合成(Mol. Cancer Ther. 2006, 5 (11), 2886),进行比较。与正甲基 43 和单甲基 44 同系物相比,CA4-偕二甲基硝基噻吩 BAPC 45 被证明是典范。它在磷酸盐缓冲液(pH 7.4,24 小时)中稳定,被 NADPH-细胞色素 P450 氧化还原酶 (POR) 裂解(25%,90 分钟),作为微管蛋白聚合抑制剂无活性(理想的前药属性)(IC50 >) 20 μM),并在 A549 细胞系中证明缺氧选择性激活 [缺氧细胞毒性比 (HCR) = 41.5]。相关的 CA1-gem-二甲基硝基噻吩 BAPC 41 也很有前景(HCR = 12.5),在 POR 处理后可完全裂解(90 分钟)。在一项初步体内动态生物发光成像研究中,BAPC 45(180 mg/kg,腹腔注射)在 4T1 同基因小鼠乳腺肿瘤模型中诱导光发射减少(4 小时内),这意味着激活和血管破坏。
更新日期:2020-03-20
中文翻译:

Combretastatin A-1 和 Combretastatin A-4 的生物还原激活前药缀合物作为针对肿瘤相关缺氧的抗癌剂。
天然产物考布他汀 A-1 (CA1) 和考布他汀 A-4 (CA4) 是微管蛋白聚合的有效抑制剂,并且是肿瘤中的选择性血管破坏剂 (VDA)。生物还原性可激活前药缀合物(BAPC)可以通过作为还原酶的底物(特别是在肿瘤缺氧区域)来增强选择性。 Davis 先前报道了一系列包含正甲基、单甲基和偕二甲基硝基噻吩触发剂的 CA1-BAPC 与相应的 CA4-BAPC 一起合成(Mol. Cancer Ther. 2006, 5 (11), 2886),进行比较。与正甲基 43 和单甲基 44 同系物相比,CA4-偕二甲基硝基噻吩 BAPC 45 被证明是典范。它在磷酸盐缓冲液(pH 7.4,24 小时)中稳定,被 NADPH-细胞色素 P450 氧化还原酶 (POR) 裂解(25%,90 分钟),作为微管蛋白聚合抑制剂无活性(理想的前药属性)(IC50 >) 20 μM),并在 A549 细胞系中证明缺氧选择性激活 [缺氧细胞毒性比 (HCR) = 41.5]。相关的 CA1-gem-二甲基硝基噻吩 BAPC 41 也很有前景(HCR = 12.5),在 POR 处理后可完全裂解(90 分钟)。在一项初步体内动态生物发光成像研究中,BAPC 45(180 mg/kg,腹腔注射)在 4T1 同基因小鼠乳腺肿瘤模型中诱导光发射减少(4 小时内),这意味着激活和血管破坏。











































 京公网安备 11010802027423号
京公网安备 11010802027423号