当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mesoporous Single-Atom-Doped Graphene–Carbon Nanotube Hybrid: Synthesis and Tunable Electrocatalytic Activity for Oxygen Evolution and Reduction Reactions
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-20 , DOI: 10.1021/acscatal.0c00352 Mohammad Tavakkoli 1 , Emmanuel Flahaut 2 , Pekka Peljo 3 , Jani Sainio 1 , Fatemeh Davodi 4 , Egor V. Lobiak 5 , Kimmo Mustonen 6 , Esko I. Kauppinen 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-20 , DOI: 10.1021/acscatal.0c00352 Mohammad Tavakkoli 1 , Emmanuel Flahaut 2 , Pekka Peljo 3 , Jani Sainio 1 , Fatemeh Davodi 4 , Egor V. Lobiak 5 , Kimmo Mustonen 6 , Esko I. Kauppinen 1
Affiliation
|
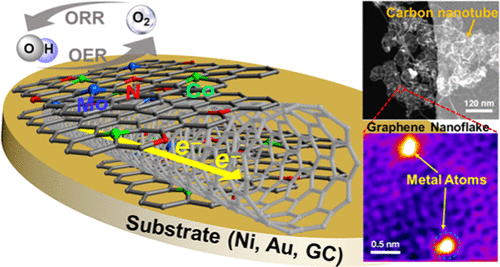
Mesoporous heteroatom-doped carbon-based nanomaterials are very promising as catalysts for electrochemical energy conversion and storage. We have developed a one-step catalytic chemical vapor deposition method to grow a highly graphitized graphene nanoflake (GF)–carbon nanotube (CNT) hybrid material doped simultaneously with single atoms of N, Co, and Mo (N–Co–Mo–GF/CNT). This high-surface-area material has a mesoporous structure, which facilitates oxygen mass transfer within the catalyst film, and exhibits a high electrocatalytic activity and stability in oxygen reduction and evolution reactions (ORR and OER) in alkaline media. We have shown that in this metal (M)–N–C catalyst, M (Co, Mo)–C centers are the main sites responsible for OER, while, for ORR, both M and N–C centers synergistically serve as the active sites. We systematically investigated tuning of the ORR and OER activity of the porous catalyst depending on the choice of the underlying substrate. The ORR kinetic current and OER activity for N–Co–Mo–GF/CNT were significantly enhanced when the catalyst was deposited onto a Ni substrate, resulting in an advanced electrocatalytic performance compared to the best bifunctional ORR/OER catalysts reported so far. Using a developed scanning electrochemical microscopy analysis method, we demonstrated that the higher OER reactivity on Ni was attributable to the formation of underlying catalyst/Ni interfacial sites, which are accessible due to the porous, electrolyte-permeable structure of the catalyst.
中文翻译:

介孔单原子掺杂石墨烯-碳纳米管杂化物:氧的释放和还原反应的合成和可调节的电催化活性
介孔杂原子掺杂的碳基纳米材料作为电化学能量转换和存储的催化剂非常有前途。我们已经开发出一种一步催化化学气相沉积方法,以生长同时掺杂有N,Co和Mo单原子的高石墨化石墨烯纳米片(GF)-碳纳米管(CNT)杂化材料(N–Co–Mo–GF / CNT)。这种高表面积材料具有中孔结构,该结构有利于氧在催化剂膜内的转移,并在碱性介质中的氧还原和放出反应(ORR和OER)中显示出高的电催化活性和稳定性。我们已经表明,在这种金属(M)–N–C催化剂中,M(Co,Mo)–C中心是负责OER的主要位点,而对于ORR,M和N–C中心都协同充当了活性中心。网站。我们根据基础底物的选择,系统地研究了多孔催化剂的ORR和OER活性的调节。当催化剂沉积到镍基体上时,N-Co-Mo-GF / CNT的ORR动力学电流和OER活性显着提高,与迄今为止报道的最佳双功能ORR / OER催化剂相比,具有先进的电催化性能。使用发达的扫描电化学显微镜分析方法,我们证明了对Ni较高的OER反应性归因于下面的催化剂/ Ni界面部位的形成,由于催化剂的多孔,电解质可渗透的结构,这些部位易于接近。当催化剂沉积到镍基体上时,N-Co-Mo-GF / CNT的ORR动力学电流和OER活性显着提高,与迄今为止报道的最佳双功能ORR / OER催化剂相比,具有先进的电催化性能。使用发达的扫描电化学显微镜分析方法,我们证明了对Ni较高的OER反应性归因于下面的催化剂/ Ni界面部位的形成,由于催化剂的多孔,电解质可渗透的结构,这些部位易于接近。当催化剂沉积到镍基体上时,N-Co-Mo-GF / CNT的ORR动力学电流和OER活性显着提高,与迄今为止报道的最佳双功能ORR / OER催化剂相比,具有先进的电催化性能。使用发达的扫描电化学显微镜分析方法,我们证明了对Ni较高的OER反应性归因于下面的催化剂/ Ni界面部位的形成,由于催化剂的多孔,电解质可渗透的结构,这些部位易于接近。
更新日期:2020-04-23
中文翻译:

介孔单原子掺杂石墨烯-碳纳米管杂化物:氧的释放和还原反应的合成和可调节的电催化活性
介孔杂原子掺杂的碳基纳米材料作为电化学能量转换和存储的催化剂非常有前途。我们已经开发出一种一步催化化学气相沉积方法,以生长同时掺杂有N,Co和Mo单原子的高石墨化石墨烯纳米片(GF)-碳纳米管(CNT)杂化材料(N–Co–Mo–GF / CNT)。这种高表面积材料具有中孔结构,该结构有利于氧在催化剂膜内的转移,并在碱性介质中的氧还原和放出反应(ORR和OER)中显示出高的电催化活性和稳定性。我们已经表明,在这种金属(M)–N–C催化剂中,M(Co,Mo)–C中心是负责OER的主要位点,而对于ORR,M和N–C中心都协同充当了活性中心。网站。我们根据基础底物的选择,系统地研究了多孔催化剂的ORR和OER活性的调节。当催化剂沉积到镍基体上时,N-Co-Mo-GF / CNT的ORR动力学电流和OER活性显着提高,与迄今为止报道的最佳双功能ORR / OER催化剂相比,具有先进的电催化性能。使用发达的扫描电化学显微镜分析方法,我们证明了对Ni较高的OER反应性归因于下面的催化剂/ Ni界面部位的形成,由于催化剂的多孔,电解质可渗透的结构,这些部位易于接近。当催化剂沉积到镍基体上时,N-Co-Mo-GF / CNT的ORR动力学电流和OER活性显着提高,与迄今为止报道的最佳双功能ORR / OER催化剂相比,具有先进的电催化性能。使用发达的扫描电化学显微镜分析方法,我们证明了对Ni较高的OER反应性归因于下面的催化剂/ Ni界面部位的形成,由于催化剂的多孔,电解质可渗透的结构,这些部位易于接近。当催化剂沉积到镍基体上时,N-Co-Mo-GF / CNT的ORR动力学电流和OER活性显着提高,与迄今为止报道的最佳双功能ORR / OER催化剂相比,具有先进的电催化性能。使用发达的扫描电化学显微镜分析方法,我们证明了对Ni较高的OER反应性归因于下面的催化剂/ Ni界面部位的形成,由于催化剂的多孔,电解质可渗透的结构,这些部位易于接近。











































 京公网安备 11010802027423号
京公网安备 11010802027423号