当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemoproteomic Identification of Serine Hydrolase RBBP9 as a Valacyclovir-Activating Enzyme.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-03-20 , DOI: 10.1021/acs.molpharmaceut.0c00131 Vikram M Shenoy 1 , Brian R Thompson 2 , Jian Shi 3 , Hao-Jie Zhu 3 , David E Smith 2 , Gordon L Amidon 2
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-03-20 , DOI: 10.1021/acs.molpharmaceut.0c00131 Vikram M Shenoy 1 , Brian R Thompson 2 , Jian Shi 3 , Hao-Jie Zhu 3 , David E Smith 2 , Gordon L Amidon 2
Affiliation
|
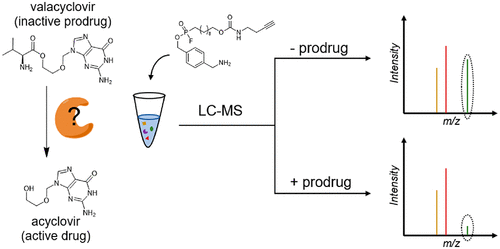
Prodrug discovery and development in the pharmaceutical industry have been hampered by a lack of knowledge of prodrug activation pathways. Such knowledge would minimize the risks of prodrug failure by enabling proper selection of preclinical animal models, prediction of pharmacogenomic variability, and identification of drug-drug interactions. Technologies for annotation of activating enzymes have not kept pace with the growing need. Activity-based protein profiling (ABPP) has matured considerably in recent decades, leading to widespread use in the pharmaceutical industry. Here, we report the extension of competitive ABPP (cABPP) to prodrug-activating enzyme identification in stable isotope-labeled cell lysates using a modified fluorophosphonate probe. Focusing on the antiviral ester prodrug valacyclovir (VACV), we identified serine hydrolase RBBP9 as an activating enzyme in Caco-2 cells via shotgun proteomics, validating the activity via the selective inhibitor emetine (EME). Kinetic characterization of RBBP9 revealed a catalytic efficiency (kcat·KM-1 = 104 mM-1·s-1) comparable to that of BPHL, the only known VACV-activating enzyme prior to this work. EME incubation in wild-type and Bphl-knockout jejunum and liver lysates demonstrated the near-exclusivity of VACV activation by RBBP9 in the intestine. Additionally, these studies showed that RBBP9 and BPHL are the two major and coequal VACV-activating enzymes in the liver. Single-pass intestinal perfusions of VACV ± EME in mice showed EME coperfusion significantly inhibited the intestinal activation of VACV, implying the in vivo relevance of RBBP9-mediated VACV activation. We envision that others might use the cABPP approach in the future for global, rapid, and efficient discovery of prodrug-activating enzymes.
中文翻译:

丝氨酸水解酶RBBP9作为Valacyclovir活化酶的化学计量学鉴定。
由于缺乏对前药活化途径的了解,阻碍了制药业中前药的发现和开发。通过使临床前动物模型的正确选择,药物基因组变异性的预测以及药物-药物相互作用的鉴定,此类知识将使前药失败的风险最小化。注释活化酶的技术未能跟上不断增长的需求。基于活动的蛋白质谱分析(ABPP)在最近几十年中已经相当成熟,从而导致了在制药工业中的广泛使用。在这里,我们报告了使用改良的氟代磷酸酯探针在稳定的同位素标记的细胞裂解物中,将竞争性ABPP(cABPP)扩展至前药激活酶的鉴定。专注于抗病毒酯前药伐昔洛韦(VACV),我们通过shot弹枪蛋白质组学鉴定了丝氨酸水解酶RBBP9作为Caco-2细胞中的活化酶,通过选择性抑制剂依美丁(EME)验证了活性。RBBP9的动力学表征显示出与BPHL相当的催化效率(kcat·KM-1 = 104 mM-1·s-1),BPHL是这项工作之前唯一已知的VACV活化酶。EME在野生型和Bphl空肠空肠和肝裂解物中的温育证明了RBBP9在肠中对VACV的激活几乎是排他的。此外,这些研究表明,RBBP9和BPHL是肝脏中两种主要且相等的VACV激活酶。小鼠VACV±EME的单次肠道灌注显示EME共灌注显着抑制VACV的肠道活化,这表明RBBP9介导的VACV活化在体内具有相关性。
更新日期:2020-03-20
中文翻译:

丝氨酸水解酶RBBP9作为Valacyclovir活化酶的化学计量学鉴定。
由于缺乏对前药活化途径的了解,阻碍了制药业中前药的发现和开发。通过使临床前动物模型的正确选择,药物基因组变异性的预测以及药物-药物相互作用的鉴定,此类知识将使前药失败的风险最小化。注释活化酶的技术未能跟上不断增长的需求。基于活动的蛋白质谱分析(ABPP)在最近几十年中已经相当成熟,从而导致了在制药工业中的广泛使用。在这里,我们报告了使用改良的氟代磷酸酯探针在稳定的同位素标记的细胞裂解物中,将竞争性ABPP(cABPP)扩展至前药激活酶的鉴定。专注于抗病毒酯前药伐昔洛韦(VACV),我们通过shot弹枪蛋白质组学鉴定了丝氨酸水解酶RBBP9作为Caco-2细胞中的活化酶,通过选择性抑制剂依美丁(EME)验证了活性。RBBP9的动力学表征显示出与BPHL相当的催化效率(kcat·KM-1 = 104 mM-1·s-1),BPHL是这项工作之前唯一已知的VACV活化酶。EME在野生型和Bphl空肠空肠和肝裂解物中的温育证明了RBBP9在肠中对VACV的激活几乎是排他的。此外,这些研究表明,RBBP9和BPHL是肝脏中两种主要且相等的VACV激活酶。小鼠VACV±EME的单次肠道灌注显示EME共灌注显着抑制VACV的肠道活化,这表明RBBP9介导的VACV活化在体内具有相关性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号