当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and Dynamics of Adrenomedullin Receptors AM1 and AM2 Reveal Key Mechanisms in the Control of Receptor Phenotype by Receptor Activity-Modifying Proteins.
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2020-03-20 , DOI: 10.1021/acsptsci.9b00080 Yi-Lynn Liang 1 , Matthew J Belousoff 1 , Madeleine M Fletcher 1 , Xin Zhang 1 , Maryam Khoshouei 2 , Giuseppe Deganutti 3 , Cassandra Koole 1 , Sebastian G B Furness 1 , Laurence J Miller 1, 4 , Debbie L Hay 5 , Arthur Christopoulos 1 , Christopher A Reynolds 3 , Radostin Danev 6 , Denise Wootten 1, 7 , Patrick M Sexton 1, 7
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2020-03-20 , DOI: 10.1021/acsptsci.9b00080 Yi-Lynn Liang 1 , Matthew J Belousoff 1 , Madeleine M Fletcher 1 , Xin Zhang 1 , Maryam Khoshouei 2 , Giuseppe Deganutti 3 , Cassandra Koole 1 , Sebastian G B Furness 1 , Laurence J Miller 1, 4 , Debbie L Hay 5 , Arthur Christopoulos 1 , Christopher A Reynolds 3 , Radostin Danev 6 , Denise Wootten 1, 7 , Patrick M Sexton 1, 7
Affiliation
|
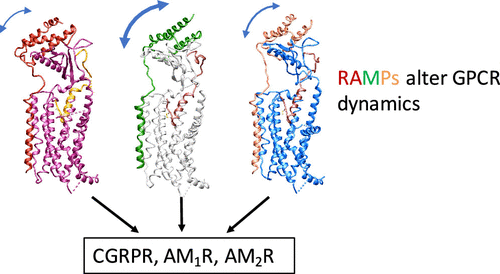
Adrenomedullin (AM) and calcitonin gene-related peptide (CGRP) receptors are critically important for metabolism, vascular tone, and inflammatory response. AM receptors are also required for normal lymphatic and blood vascular development and angiogenesis. They play a pivotal role in embryo implantation and fertility and can provide protection against hypoxic and oxidative stress. CGRP and AM receptors are heterodimers of the calcitonin receptor-like receptor (CLR) and receptor activity-modifying protein 1 (RAMP1) (CGRPR), as well as RAMP2 or RAMP3 (AM1R and AM2R, respectively). However, the mechanistic basis for RAMP modulation of CLR phenotype is unclear. In this study, we report the cryo-EM structure of the AM1R in complex with AM and Gs at a global resolution of 3.0 Å, and structures of the AM2R in complex with either AM or intermedin/adrenomedullin 2 (AM2) and Gs at 2.4 and 2.3 Å, respectively. The structures reveal distinctions in the primary orientation of the extracellular domains (ECDs) relative to the receptor core and distinct positioning of extracellular loop 3 (ECL3) that are receptor-dependent. Analysis of dynamic data present in the cryo-EM micrographs revealed additional distinctions in the extent of mobility of the ECDs. Chimeric exchange of the linker region of the RAMPs connecting the TM helix and the ECD supports a role for this segment in controlling receptor phenotype. Moreover, a subset of the motions of the ECD appeared coordinated with motions of the G protein relative to the receptor core, suggesting that receptor ECD dynamics could influence G protein interactions. This work provides fundamental advances in our understanding of GPCR function and how this can be allosterically modulated by accessory proteins.
中文翻译:

肾上腺髓质素受体 AM1 和 AM2 的结构和动力学揭示了受体活性修饰蛋白控制受体表型的关键机制。
肾上腺髓质素 (AM) 和降钙素基因相关肽 (CGRP) 受体对于新陈代谢、血管张力和炎症反应至关重要。 AM 受体也是正常淋巴管和血管发育及血管生成所必需的。它们在胚胎植入和生育能力中发挥着关键作用,可以提供针对缺氧和氧化应激的保护。 CGRP 和 AM 受体是降钙素受体样受体 (CLR) 和受体活性修饰蛋白 1 (RAMP1) (CGRPR) 以及 RAMP2 或 RAMP3(分别为 AM1R 和 AM2R)的异二聚体。然而,RAMP 调节 CLR 表型的机制基础尚不清楚。在本研究中,我们报告了 AM1R 与 AM 和 Gs 复合物的冷冻电镜结构,全局分辨率为 3.0 Å,以及 AM2R 与 AM 或中间体/肾上腺髓质素 2 (AM2) 和 Gs 复合物的冷冻电镜结构,分辨率为 2.4 Å。分别为 2.3 和 2.3 埃。这些结构揭示了细胞外结构域 (ECD) 相对于受体核心的主要方向的差异以及受体依赖性细胞外环 3 (ECL3) 的独特定位。对冷冻电镜显微照片中的动态数据的分析揭示了 ECD 的移动程度的额外区别。连接 TM 螺旋和 ECD 的 RAMP 连接区的嵌合交换支持了该片段在控制受体表型中的作用。此外,ECD 运动的一部分似乎与 G 蛋白相对于受体核心的运动协调,表明受体 ECD 动力学可能影响 G 蛋白相互作用。这项工作为我们理解 GPCR 功能以及如何通过辅助蛋白进行变构调节提供了基础性进展。
更新日期:2020-04-23
中文翻译:

肾上腺髓质素受体 AM1 和 AM2 的结构和动力学揭示了受体活性修饰蛋白控制受体表型的关键机制。
肾上腺髓质素 (AM) 和降钙素基因相关肽 (CGRP) 受体对于新陈代谢、血管张力和炎症反应至关重要。 AM 受体也是正常淋巴管和血管发育及血管生成所必需的。它们在胚胎植入和生育能力中发挥着关键作用,可以提供针对缺氧和氧化应激的保护。 CGRP 和 AM 受体是降钙素受体样受体 (CLR) 和受体活性修饰蛋白 1 (RAMP1) (CGRPR) 以及 RAMP2 或 RAMP3(分别为 AM1R 和 AM2R)的异二聚体。然而,RAMP 调节 CLR 表型的机制基础尚不清楚。在本研究中,我们报告了 AM1R 与 AM 和 Gs 复合物的冷冻电镜结构,全局分辨率为 3.0 Å,以及 AM2R 与 AM 或中间体/肾上腺髓质素 2 (AM2) 和 Gs 复合物的冷冻电镜结构,分辨率为 2.4 Å。分别为 2.3 和 2.3 埃。这些结构揭示了细胞外结构域 (ECD) 相对于受体核心的主要方向的差异以及受体依赖性细胞外环 3 (ECL3) 的独特定位。对冷冻电镜显微照片中的动态数据的分析揭示了 ECD 的移动程度的额外区别。连接 TM 螺旋和 ECD 的 RAMP 连接区的嵌合交换支持了该片段在控制受体表型中的作用。此外,ECD 运动的一部分似乎与 G 蛋白相对于受体核心的运动协调,表明受体 ECD 动力学可能影响 G 蛋白相互作用。这项工作为我们理解 GPCR 功能以及如何通过辅助蛋白进行变构调节提供了基础性进展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号