当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
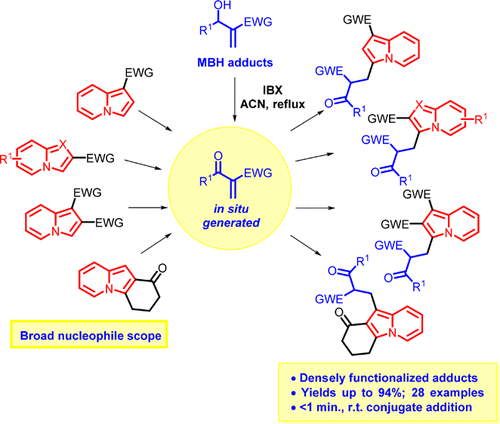
Catalyst-Free Conjugate Addition of Indolizines to In Situ-Generated Oxidized Morita–Baylis–Hillman Adducts
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-03-20 , DOI: 10.1021/acs.joc.0c00189 Thiago S. Silva 1 , Lucas A. Zeoly 1 , Fernando Coelho 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-03-20 , DOI: 10.1021/acs.joc.0c00189 Thiago S. Silva 1 , Lucas A. Zeoly 1 , Fernando Coelho 1
Affiliation
|
Sequential one-pot 2-iodoxybenzoic acid (IBX) oxidation of Morita–Baylis–Hillman (MBH) adducts followed by catalyst-free indolizine conjugate addition was developed. The wide scopes of MBH adducts and indolizines were investigated, and densely functionalized adducts were obtained in yields of up to 94%. The conjugate addition step occurred in less than a minute at room temperature with total regioselectivity toward indolizine C3 carbon. Less nucleophilic C1 carbon was also alkylated when C3-substituted indolizines were employed as the substrate.
中文翻译:

吲哚胺类化合物在原位生成的氧化森田-贝利斯-希尔曼加合物上无催化剂的共轭加成反应
开发了森田-贝利斯-希尔曼(MBH)加合物的顺序一锅法2-碘氧基苯甲酸(IBX)氧化,然后加入无催化剂的吲哚嗪共轭物。研究了广泛的MBH加合物和吲哚嗪类化合物,并获得了功能密集的加合物,收率高达94%。缀合物添加步骤在室温下不到一分钟的时间内发生,对吲哚嗪C3碳的总区域选择性高。当使用C3取代的吲哚嗪作为底物时,较少的亲核C1碳也被烷基化。
更新日期:2020-04-24
中文翻译:

吲哚胺类化合物在原位生成的氧化森田-贝利斯-希尔曼加合物上无催化剂的共轭加成反应
开发了森田-贝利斯-希尔曼(MBH)加合物的顺序一锅法2-碘氧基苯甲酸(IBX)氧化,然后加入无催化剂的吲哚嗪共轭物。研究了广泛的MBH加合物和吲哚嗪类化合物,并获得了功能密集的加合物,收率高达94%。缀合物添加步骤在室温下不到一分钟的时间内发生,对吲哚嗪C3碳的总区域选择性高。当使用C3取代的吲哚嗪作为底物时,较少的亲核C1碳也被烷基化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号