当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spiro-Indole-Coumarin Hybrids: Synthesis, ADME, DFT, NBO Studies and In Silico Screening through Molecular Docking on DNA G-Quadruplex.
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-03-19 , DOI: 10.1002/slct.201904783 Leena Khanna 1, 2 , Sugandha Singhal 2 , Subhash C Jain 1 , Pankaj Khanna 1, 3
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-03-19 , DOI: 10.1002/slct.201904783 Leena Khanna 1, 2 , Sugandha Singhal 2 , Subhash C Jain 1 , Pankaj Khanna 1, 3
Affiliation
|
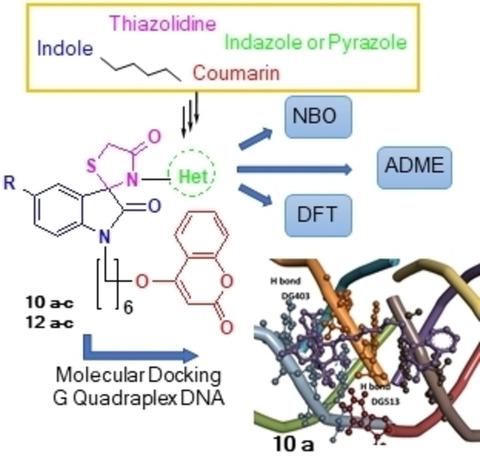
New series of hybrids were synthesized by combination of 4-hydroxycoumarin with spiro[indol-indazole-thiazolidine]-diones and spiro[indol-pyrazole-thiazolidine]-diones, via hitherto unknown Schiff bases. The effects of substituents, such as -F, -Br and -CH3, on the crucial characteristics pertaining to the hybrids were investigated through computational studies. In silico or virtual screening through molecular docking studies on the library of 22 compounds, including reference compounds, precursors, non-hybrid and hybrid derivatives, was performed on DNA G-quadruplex of the human genome. All six freshly synthesized hybrids showed high binding energy as compared to non-hybrids as well as reference compounds. The presence of substituents at 5-position of indole enhanced the binding tendency of the ligand. ADME studies indicated good oral bioavailability and absorption of these compounds. Density Functional Theory (DFT) calculations of hybrids were done at B3LYP/6-311G++(d,p) level of computation. Their HOMO and LUMO energy plots reflected the presence of high charge transfer and chemical potential. Natural bond order (NBO) calculations predicted hyperconjugative interactions. The Molecular Electrostatic Potential (MEP) surface plots showed possible electrophilic and nucleophilic attacking sites of the hybrids. Compound 10 a (5-fluoro-spiro[indol-indazole-thiazolidine]-dione-coumarin hybrid), on the basis of global reactivity descriptors, was filtered to be chemically most reactive with the highest binding energy of -8.23 kcal/mol with DNA G-quadruplex. The synthesized hybrid coumarin derivatives in correlation with theoretical docking studies validate that hybrid derivatives are more reactive compared to their non-hybrid counterparts.
中文翻译:

螺-吲哚-香豆素杂化物:通过 DNA G-四链体上的分子对接进行合成、ADME、DFT、NBO 研究和计算机筛选。
通过将 4-羟基香豆素与螺[吲哚-吲唑-噻唑烷]-二酮和螺[吲哚-吡唑-噻唑烷]-二酮通过迄今未知的席夫碱组合,合成了新系列的杂化物。通过计算研究研究了取代基(例如-F、-Br和-CH3)对杂种关键特性的影响。通过分子对接研究对人类基因组 DNA G-四链体的 22 种化合物库(包括参考化合物、前体、非杂交和杂交衍生物)进行了计算机或虚拟筛选。与非杂化物以及参考化合物相比,所有六种新合成的杂化物都显示出高结合能。吲哚5位取代基的存在增强了配体的结合倾向。 ADME 研究表明这些化合物具有良好的口服生物利用度和吸收性。混合体的密度泛函理论 (DFT) 计算在 B3LYP/6-311G++(d,p) 计算级别完成。他们的 HOMO 和 LUMO 能量图反映了高电荷转移和化学势的存在。自然键序 (NBO) 计算预测了超共轭相互作用。分子静电势(MEP)表面图显示了杂化物可能的亲电和亲核攻击位点。化合物 10a(5-氟-螺[吲哚-吲唑-噻唑烷]-二酮-香豆素杂化物),基于全局反应性描述符,被筛选为化学反应性最强的化合物,其最高结合能为-8.23 kcal/mol DNA G-四链体。合成的杂化香豆素衍生物与理论对接研究相关,证实杂化衍生物比非杂化衍生物更具反应性。
更新日期:2020-03-20
中文翻译:

螺-吲哚-香豆素杂化物:通过 DNA G-四链体上的分子对接进行合成、ADME、DFT、NBO 研究和计算机筛选。
通过将 4-羟基香豆素与螺[吲哚-吲唑-噻唑烷]-二酮和螺[吲哚-吡唑-噻唑烷]-二酮通过迄今未知的席夫碱组合,合成了新系列的杂化物。通过计算研究研究了取代基(例如-F、-Br和-CH3)对杂种关键特性的影响。通过分子对接研究对人类基因组 DNA G-四链体的 22 种化合物库(包括参考化合物、前体、非杂交和杂交衍生物)进行了计算机或虚拟筛选。与非杂化物以及参考化合物相比,所有六种新合成的杂化物都显示出高结合能。吲哚5位取代基的存在增强了配体的结合倾向。 ADME 研究表明这些化合物具有良好的口服生物利用度和吸收性。混合体的密度泛函理论 (DFT) 计算在 B3LYP/6-311G++(d,p) 计算级别完成。他们的 HOMO 和 LUMO 能量图反映了高电荷转移和化学势的存在。自然键序 (NBO) 计算预测了超共轭相互作用。分子静电势(MEP)表面图显示了杂化物可能的亲电和亲核攻击位点。化合物 10a(5-氟-螺[吲哚-吲唑-噻唑烷]-二酮-香豆素杂化物),基于全局反应性描述符,被筛选为化学反应性最强的化合物,其最高结合能为-8.23 kcal/mol DNA G-四链体。合成的杂化香豆素衍生物与理论对接研究相关,证实杂化衍生物比非杂化衍生物更具反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号