当前位置:
X-MOL 学术
›
Plasma Processes Polym.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A chemical-kinetic model of DBDs in Ar-H2 O mixtures
Plasma Processes and Polymers ( IF 2.9 ) Pub Date : 2020-03-19 , DOI: 10.1002/ppap.202000028 Claus‐Peter Klages 1
Plasma Processes and Polymers ( IF 2.9 ) Pub Date : 2020-03-19 , DOI: 10.1002/ppap.202000028 Claus‐Peter Klages 1
Affiliation
|
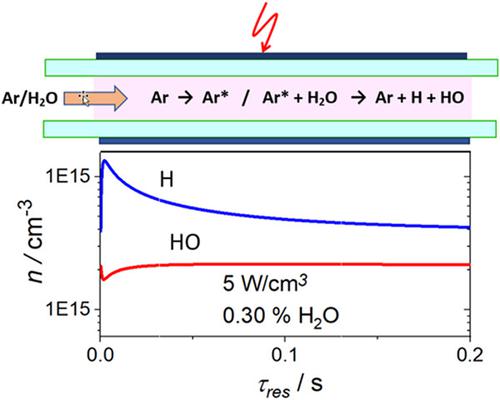
Funding information Federal Ministry of Education and Research (BMBF), Grant/Award Number: FKZ: 01DQ16011A Abstract A simplified chemical‐kinetic model was applied to Ar‐H2O dielectric‐barrier discharges (DBDs), presuming that dissociation processes are only due to energy transfer from excited Ar species. Good agreement was obtained between the densities of HO, H2, and O2 and experimental data from the literature, whereas a discrepancy for H2O2 could not be explained. The model is useful for designing DBD reactors and process development. Steady‐state densities of H atoms increase with decreasing fractions of xH O 2 which should be kept below 0.1% to obtain a large zone of virtually constant and large H‐atom density in the DBD reactor, whereas the HO density is hardly affected by xH O 2 . O2 contaminations must be kept well below 100 ppm in to attain maximum H‐atom densities.
中文翻译:

Ar-H2 O 混合物中 DBD 的化学动力学模型
资金信息 联邦教育和研究部 (BMBF),资助/奖励编号:FKZ:01DQ16011A 摘要 将简化的化学动力学模型应用于 Ar-H2O 介电势垒放电 (DBD),假设解离过程仅由能量引起从激发的 Ar 物种转移。H2O、H2 和 O2 的密度与文献中的实验数据之间获得了良好的一致性,而 H2O2 的差异无法解释。该模型可用于设计 DBD 反应器和工艺开发。H 原子的稳态密度随着 xH O 2 分数的减少而增加,应保持在 0.1% 以下以获得 DBD 反应器中几乎恒定和大 H 原子密度的大区域,而 H2O 密度几乎不受 xH 的影响○ 2 。
更新日期:2020-03-19
中文翻译:

Ar-H2 O 混合物中 DBD 的化学动力学模型
资金信息 联邦教育和研究部 (BMBF),资助/奖励编号:FKZ:01DQ16011A 摘要 将简化的化学动力学模型应用于 Ar-H2O 介电势垒放电 (DBD),假设解离过程仅由能量引起从激发的 Ar 物种转移。H2O、H2 和 O2 的密度与文献中的实验数据之间获得了良好的一致性,而 H2O2 的差异无法解释。该模型可用于设计 DBD 反应器和工艺开发。H 原子的稳态密度随着 xH O 2 分数的减少而增加,应保持在 0.1% 以下以获得 DBD 反应器中几乎恒定和大 H 原子密度的大区域,而 H2O 密度几乎不受 xH 的影响○ 2 。











































 京公网安备 11010802027423号
京公网安备 11010802027423号