当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NixFe1−xB nanoparticle self-modified nanosheets as efficient bifunctional electrocatalysts for water splitting: experiments and theories
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2020/03/19 , DOI: 10.1039/c9ta14058a Weizhao Hong 1, 2, 3, 4, 5 , Shanfu Sun 1, 2, 3, 4, 5 , Yi Kong 1, 2, 3, 4, 5 , Yongyuan Hu 1, 2, 3, 4, 5 , Gang Chen 1, 2, 3, 4, 5
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2020/03/19 , DOI: 10.1039/c9ta14058a Weizhao Hong 1, 2, 3, 4, 5 , Shanfu Sun 1, 2, 3, 4, 5 , Yi Kong 1, 2, 3, 4, 5 , Yongyuan Hu 1, 2, 3, 4, 5 , Gang Chen 1, 2, 3, 4, 5
Affiliation
|
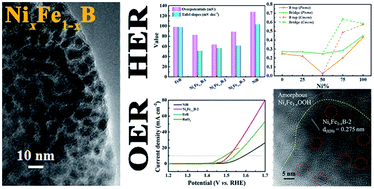
Developing highly efficient, stable, and inexpensive electrocatalysts for both the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER) is the premise of achieving large-scale water splitting. Here, NixFe1−xB prepared by a facile borothermal reduction in molten salt is a robust bifunctional electrocatalyst for overall water splitting. The NixFe1−xB electrocatalyst achieves a current density of 10 mA cm−2 at overpotentials of 63.5 mV for the HER and 282 mV for the OER in 1 M KOH electrolyte, respectively. Density functional theory (DFT) calculations suggest that the NixFe1−xB possesses a lower absolute value of H* adsorption free energy (|ΔGH*|) than the primitive FeB and NiB, which is beneficial to improve the HER activity. Two identical NixFe1−xB electrodes are combined to form an alkaline electrolyzer, which achieves a current density of 10 mA cm−2 at 1.57 V for overall water splitting. This work provides a strategy to develop highly efficient transition metal boride catalysts for overall water splitting.
中文翻译:

NixFe1-xB纳米粒子自修饰纳米片作为高效的水分解双功能电催化剂:实验和理论
开发用于氢气析出反应(HER)和氧气析出反应(OER)的高效,稳定且廉价的电催化剂是实现大规模水分解的前提。在此,通过在熔融盐中进行轻松的硼热还原制得的Ni x Fe 1- x B是用于整体水分解的稳健的双功能电催化剂。在1 M KOH电解质中,HER的超电势和OER的282 mV的超电势分别使Ni x Fe 1- x B电催化剂达到10 mA cm -2的电流密度。密度泛函理论(DFT)计算表明Ni x Fe 1- xB具有比原始FeB和NiB更低的H *吸附自由能绝对值(| ΔG H * |),这有利于提高HER活性。将两个相同的Ni x Fe 1- x B电极组合以形成碱性电解槽,该碱性电解槽在1.57 V的电流下实现了10 mA cm -2的电流密度,从而实现了总的水分解。这项工作提供了开发用于整体水分解的高效过渡金属硼化物催化剂的策略。
更新日期:2020-04-15
中文翻译:

NixFe1-xB纳米粒子自修饰纳米片作为高效的水分解双功能电催化剂:实验和理论
开发用于氢气析出反应(HER)和氧气析出反应(OER)的高效,稳定且廉价的电催化剂是实现大规模水分解的前提。在此,通过在熔融盐中进行轻松的硼热还原制得的Ni x Fe 1- x B是用于整体水分解的稳健的双功能电催化剂。在1 M KOH电解质中,HER的超电势和OER的282 mV的超电势分别使Ni x Fe 1- x B电催化剂达到10 mA cm -2的电流密度。密度泛函理论(DFT)计算表明Ni x Fe 1- xB具有比原始FeB和NiB更低的H *吸附自由能绝对值(| ΔG H * |),这有利于提高HER活性。将两个相同的Ni x Fe 1- x B电极组合以形成碱性电解槽,该碱性电解槽在1.57 V的电流下实现了10 mA cm -2的电流密度,从而实现了总的水分解。这项工作提供了开发用于整体水分解的高效过渡金属硼化物催化剂的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号