Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Loss of the nuclear Wnt pathway effector TCF7L2 promotes migration and invasion of human colorectal cancer cells.
Oncogene ( IF 6.9 ) Pub Date : 2020-03-20 , DOI: 10.1038/s41388-020-1259-7 Janna Wenzel 1, 2 , Katja Rose 1 , Elham Bavafaye Haghighi 3 , Constanze Lamprecht 4, 5, 6 , Gilles Rauen 2, 5 , Vivien Freihen 1 , Rebecca Kesselring 7 , Melanie Boerries 3, 8, 9 , Andreas Hecht 1, 2, 5
Oncogene ( IF 6.9 ) Pub Date : 2020-03-20 , DOI: 10.1038/s41388-020-1259-7 Janna Wenzel 1, 2 , Katja Rose 1 , Elham Bavafaye Haghighi 3 , Constanze Lamprecht 4, 5, 6 , Gilles Rauen 2, 5 , Vivien Freihen 1 , Rebecca Kesselring 7 , Melanie Boerries 3, 8, 9 , Andreas Hecht 1, 2, 5
Affiliation
|
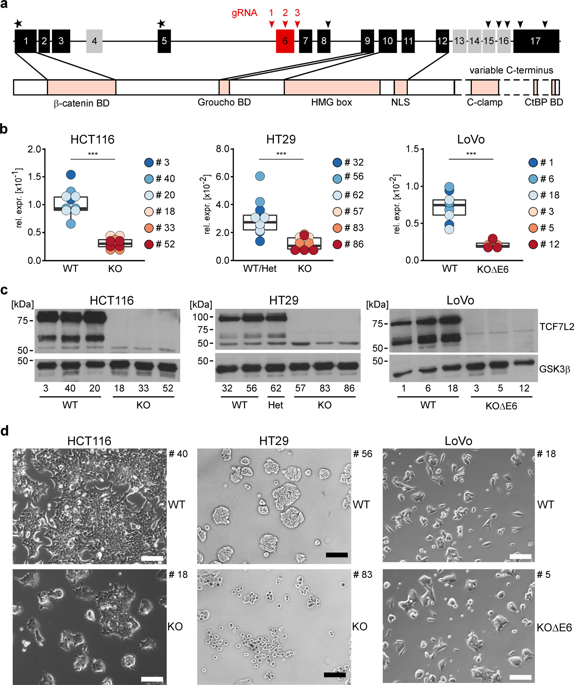
The transcription factor TCF7L2 is indispensable for intestinal tissue homeostasis where it transmits mitogenic Wnt/β-Catenin signals in stem and progenitor cells, from which intestinal tumors arise. Yet, TCF7L2 belongs to the most frequently mutated genes in colorectal cancer (CRC), and tumor-suppressive functions of TCF7L2 were proposed. This apparent paradox warrants to clarify the role of TCF7L2 in colorectal carcinogenesis. Here, we investigated TCF7L2 dependence/independence of CRC cells and the cellular and molecular consequences of TCF7L2 loss-of-function. By genome editing we achieved complete TCF7L2 inactivation in several CRC cell lines without loss of viability, showing that CRC cells have widely lost the strict requirement for TCF7L2. TCF7L2 deficiency impaired G1/S progression, reminiscent of the physiological role of TCF7L2. In addition, TCF7L2-negative cells exhibited morphological changes, enhanced migration, invasion, and collagen adhesion, albeit the severity of the phenotypic alterations manifested in a cell-line-specific fashion. To provide a molecular framework for the observed cellular changes, we performed global transcriptome profiling and identified gene-regulatory networks in which TCF7L2 positively regulates the proto-oncogene MYC, while repressing the cell cycle inhibitors CDKN2C/CDKN2D. Consistent with its function in curbing cell motility and invasion, TCF7L2 directly suppresses the pro-metastatic transcription factor RUNX2 and impinges on the expression of cell adhesion molecules. Altogether, we conclude that the proliferation-stimulating activity of TCF7L2 persists in CRC cells. In addition, TCF7L2 acts as invasion suppressor. Despite its negative impact on cell cycle progression, TCF7L2 loss-of-function may thereby increase malignancy, which could explain why TCF7L2 is mutated in a sizeable fraction of colorectal tumors.
中文翻译:

核Wnt途径效应子TCF7L2的丧失促进了人类结直肠癌细胞的迁移和侵袭。
转录因子TCF7L2对于肠道组织稳态是必不可少的,它在干细胞和祖细胞中传递有丝分裂性Wnt /β-Catenin信号,从而产生肠道肿瘤。然而,TCF7L2属于大肠癌(CRC)中最频繁突变的基因,并且提出了TCF7L2的肿瘤抑制功能。这种明显的悖论有必要阐明TCF7L2在结直肠癌发生中的作用。在这里,我们研究了TCF7L2对CRC细胞的依赖性/独立性以及TCF7L2功能丧失的细胞和分子后果。通过基因组编辑,我们在几种CRC细胞系中实现了TCF7L2的完全失活,而没有丧失生存能力,这表明CRC细胞已广泛失去了对TCF7L2的严格要求。TCF7L2缺乏会损害G1 / S进程,让人联想到TCF7L2的生理作用。此外,TCF7L2阴性细胞表现出形态变化,迁移,侵袭和胶原蛋白粘附增强,尽管表型改变的严重性以细胞系特异性方式表现。为了提供观察到的细胞变化的分子框架,我们进行了整体转录组分析,并确定了基因调控网络,其中TCF7L2积极调控原癌基因MYC,同时抑制细胞周期抑制剂CDKN2C / CDKN2D。与它在抑制细胞运动和侵袭中的功能一致,TCF7L2直接抑制促转移转录因子RUNX2并影响细胞粘附分子的表达。总之,我们得出结论,TCF7L2的增殖刺激活性在CRC细胞中持续存在。另外,TCF7L2充当入侵抑制器。
更新日期:2020-03-20
中文翻译:

核Wnt途径效应子TCF7L2的丧失促进了人类结直肠癌细胞的迁移和侵袭。
转录因子TCF7L2对于肠道组织稳态是必不可少的,它在干细胞和祖细胞中传递有丝分裂性Wnt /β-Catenin信号,从而产生肠道肿瘤。然而,TCF7L2属于大肠癌(CRC)中最频繁突变的基因,并且提出了TCF7L2的肿瘤抑制功能。这种明显的悖论有必要阐明TCF7L2在结直肠癌发生中的作用。在这里,我们研究了TCF7L2对CRC细胞的依赖性/独立性以及TCF7L2功能丧失的细胞和分子后果。通过基因组编辑,我们在几种CRC细胞系中实现了TCF7L2的完全失活,而没有丧失生存能力,这表明CRC细胞已广泛失去了对TCF7L2的严格要求。TCF7L2缺乏会损害G1 / S进程,让人联想到TCF7L2的生理作用。此外,TCF7L2阴性细胞表现出形态变化,迁移,侵袭和胶原蛋白粘附增强,尽管表型改变的严重性以细胞系特异性方式表现。为了提供观察到的细胞变化的分子框架,我们进行了整体转录组分析,并确定了基因调控网络,其中TCF7L2积极调控原癌基因MYC,同时抑制细胞周期抑制剂CDKN2C / CDKN2D。与它在抑制细胞运动和侵袭中的功能一致,TCF7L2直接抑制促转移转录因子RUNX2并影响细胞粘附分子的表达。总之,我们得出结论,TCF7L2的增殖刺激活性在CRC细胞中持续存在。另外,TCF7L2充当入侵抑制器。











































 京公网安备 11010802027423号
京公网安备 11010802027423号