Molecular Therapy - Nucleic Acids ( IF 6.5 ) Pub Date : 2020-03-19 , DOI: 10.1016/j.omtn.2020.03.005 Yu-Chan Yang , Yu-Hsiang Chen , Jia-Horng Kao , Chi Ching , I-Jung Liu , Chih-Chiang Wang , Cheng-Hsueh Tsai , Fang-Yi Wu , Chun-Jen Liu , Pei-Jer Chen , Ding-Shinn Chen , Hung-Chih Yang
|
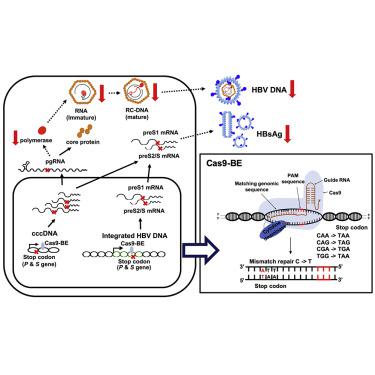
Current antiviral therapy fails to cure chronic hepatitis B virus (HBV) infection because of persistent covalently closed circular DNA (cccDNA). CRISPR/Cas9-mediated specific cleavage of cccDNA is a potentially curative strategy for chronic hepatitis B (CHB). However, the CRISPR/Cas system inevitably targets integrated HBV DNA and induces double-strand breaks (DSBs) of host genome, bearing the risk of genomic rearrangement and damage. Herein, we examined the utility of recently developed CRISPR/Cas-mediated “base editors” (BEs) in inactivating HBV gene expression without cleavage of DNA. Candidate target sites of the SpCas9-derived BE and its variants in HBV genomes were screened for generating nonsense mutations of viral genes with individual guide RNAs (gRNAs). SpCas9-BE with certain gRNAs effectively base-edited polymerase and surface genes and reduced HBV gene expression in cells harboring integrated HBV genomes, but induced very few insertions or deletions (indels). Interestingly, some point mutations introduced by base editing resulted in simultaneous suppression of both polymerase and surface genes. Finally, the episomal cccDNA was successfully edited by SpCas9-BE for suppression of viral gene expression in an in vitro HBV infection system. In conclusion, Cas9-mediated base editing is a potential strategy to cure CHB by permanent inactivation of integrated HBV DNA and cccDNA without DSBs of the host genome.
中文翻译:

通过CRISPR / Cas9介导的非切割碱基编辑永久灭活HBV基因组。
由于持续的共价闭合环状DNA(cccDNA),当前的抗病毒治疗无法治愈慢性乙型肝炎病毒(HBV)感染。CRISPR / Cas9介导的cccDNA特异性切割是治疗慢性乙型肝炎(CHB)的潜在策略。然而,CRISPR / Cas系统不可避免地靶向整合的HBV DNA,并诱导宿主基因组的双链断裂(DSB),承担着基因组重排和破坏的风险。本文中,我们研究了最近开发的CRISPR / Cas介导的“碱基编辑器”(BEs)在不切割DNA的情况下灭活HBV基因表达的效用。筛选了SpCas9衍生的BE及其在HBV基因组中的变体的候选靶位点,以产生带有单个指导RNA(gRNA)的病毒基因的无义突变。具有某些gRNA的SpCas9-BE有效地编辑了聚合酶和表面基因,并降低了带有整合HBV基因组的细胞中HBV基因的表达,但几乎没有插入或缺失(indels)。有趣的是,碱基编辑引入的一些点突变导致同时抑制聚合酶和表面基因。最后,由SpCas9-BE成功编辑了附加型cccDNA,用于抑制病毒基因的表达。体外HBV感染系统。总之,Cas9介导的碱基编辑是通过永久灭活整合的HBV DNA和cccDNA而没有宿主基因组DSB的方法来治愈CHB的潜在策略。










































 京公网安备 11010802027423号
京公网安备 11010802027423号